Embedded 3D Photopatterning of Hydrogels with Diverse and Complex Architectures for Tissue Engineering and Disease Models
- PMID: 26154197
- PMCID: PMC4638205
- DOI: 10.1089/ten.TEC.2015.0179
Embedded 3D Photopatterning of Hydrogels with Diverse and Complex Architectures for Tissue Engineering and Disease Models
Abstract
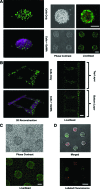
Techniques that can create three-dimensional (3D) structures to provide architectural support for cells have a significant impact in generating complex and hierarchically organized tissues/organs. In recent times, a number of technologies, including photopatterning, have been developed to create such intricate 3D structures. In this study, we describe an easy-to-implement photopatterning approach, involving a conventional fluorescent microscope and a simple photomask, to encapsulate cells within spatially defined 3D structures. We have demonstrated the ease and the versatility of this approach by creating simple to complex as well as multilayered structures. We have extended this photopatterning approach to incorporate and spatially organize multiple cell types, thereby establishing coculture systems. Such cost-effective and easy-to-use approaches can greatly advance tissue engineering strategies.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

