Geraniol Suppresses Angiogenesis by Downregulating Vascular Endothelial Growth Factor (VEGF)/VEGFR-2 Signaling
- PMID: 26154255
- PMCID: PMC4496091
- DOI: 10.1371/journal.pone.0131946
Geraniol Suppresses Angiogenesis by Downregulating Vascular Endothelial Growth Factor (VEGF)/VEGFR-2 Signaling
Abstract
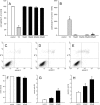
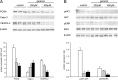
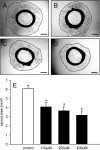
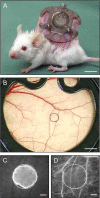
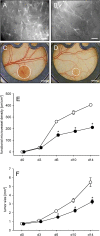
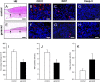
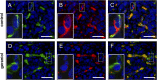
Geraniol exerts several direct pharmacological effects on tumor cells and, thus, has been suggested as a promising anti-cancer compound. Because vascularization is a major precondition for tumor growth, we analyzed in this study the anti-angiogenic action of geraniol. In vitro, geraniol reduced the migratory activity of endothelial-like eEND2 cells. Western blot analyses further revealed that geraniol downregulates proliferating cell nuclear antigen (PCNA) and upregulates cleaved caspase-3 (Casp-3) expression in eEND2 cells. Moreover, geraniol blocked vascular endothelial growth factor (VEGF)/VEGFR-2 signal transduction, resulting in a suppression of downstream AKT and ERK signaling pathways. In addition, geraniol significantly reduced vascular sprout formation in a rat aortic ring assay. In vivo, geraniol inhibited the vascularization of CT26 tumors in dorsal skinfold chambers of BALB/c mice, which was associated with a smaller tumor size when compared to vehicle-treated controls. Immunohistochemical analyses confirmed a decreased number of Ki67-positive cells and CD31-positive microvessels with reduced VEGFR-2 expression within geraniol-treated tumors. Taken together, these findings indicate that geraniol targets multiple angiogenic mechanisms and, therefore, is an attractive candidate for the anti-angiogenic treatment of tumors.
Conflict of interest statement
Figures


Similar articles
-
Inhibition of angiogenesis: a novel antitumor mechanism of the herbal compound arctigenin.Anticancer Drugs. 2013 Sep;24(8):781-91. doi: 10.1097/CAD.0b013e328362fb84. Anticancer Drugs. 2013. PMID: 23744558
-
Inhibition of Hyaluronic Acid Synthesis Suppresses Angiogenesis in Developing Endometriotic Lesions.PLoS One. 2016 Mar 28;11(3):e0152302. doi: 10.1371/journal.pone.0152302. eCollection 2016. PLoS One. 2016. PMID: 27018976 Free PMC article.
-
ZLM-7 exhibits anti-angiogenic effects via impaired endothelial cell function and blockade of VEGF/VEGFR-2 signaling.Oncotarget. 2016 Apr 5;7(14):19018-30. doi: 10.18632/oncotarget.7968. Oncotarget. 2016. PMID: 26967559 Free PMC article.
-
The antitumor effects of geraniol: Modulation of cancer hallmark pathways (Review).Int J Oncol. 2016 May;48(5):1772-82. doi: 10.3892/ijo.2016.3427. Epub 2016 Mar 9. Int J Oncol. 2016. PMID: 26983575 Free PMC article. Review.
-
Pharmacological Properties of Geraniol - A Review.Planta Med. 2019 Jan;85(1):48-55. doi: 10.1055/a-0750-6907. Epub 2018 Oct 11. Planta Med. 2019. PMID: 30308694 Review.
Cited by
-
One Hundred Faces of Geraniol.Molecules. 2020 Jul 21;25(14):3303. doi: 10.3390/molecules25143303. Molecules. 2020. PMID: 32708169 Free PMC article. Review.
-
Geraniol attenuates hydrogen peroxide-induced liver fatty acid alterations in male rats.J Intercult Ethnopharmacol. 2016 Oct 4;6(1):29-35. doi: 10.5455/jice.20160928012410. eCollection 2017 Jan-Mar. J Intercult Ethnopharmacol. 2016. PMID: 28163957 Free PMC article.
-
Preventive Effect of Geraniol on Diethylnitrosamine-Induced Hepatocarcinogenesis in Rats.Yonago Acta Med. 2016 Mar;59(1):37-43. Epub 2016 Apr 1. Yonago Acta Med. 2016. PMID: 27046949 Free PMC article.
-
Phytochemicals Showing Antiangiogenic Effect in Pre-clinical Models and their Potential as an Alternative to Existing Therapeutics.Curr Top Med Chem. 2024;24(4):259-300. doi: 10.2174/0115680266264349231016094456. Curr Top Med Chem. 2024. PMID: 37867279 Review.
-
The Aortic Ring Co-culture Assay: A Convenient Tool to Assess the Angiogenic Potential of Mesenchymal Stromal Cells In Vitro.J Vis Exp. 2017 Sep 18;(127):56083. doi: 10.3791/56083. J Vis Exp. 2017. PMID: 28994816 Free PMC article.
References
-
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29: 15–18. - PubMed
-
- Rak JW, St Croix BD, Kerbel RS. Consequences of angiogenesis for tumor progression, metastasis and cancer therapy. Anticancer Drugs 1995;6: 3–18. - PubMed
-
- Lauro S, Onesti CE, Righini R, Marchetti P. The use of bevacizumab in non-small cell lung cancer: an update. Anticancer Res. 2014;34: 1537–1545. - PubMed
-
- Khan AQ, Khan R, Qamar W, Lateef A, Rehman MU, Tahir M, et al. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: possible role of p38 MAP Kinase and NF-κB. Exp Mol Pathol. 2013;94: 419–429. 10.1016/j.yexmp.2013.01.006 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

