Systemic siRNA Nanoparticle-Based Drugs Combined with Radiofrequency Ablation for Cancer Therapy
- PMID: 26154425
- PMCID: PMC4495977
- DOI: 10.1371/journal.pone.0128910
Systemic siRNA Nanoparticle-Based Drugs Combined with Radiofrequency Ablation for Cancer Therapy
Abstract
Purpose: Radiofrequency thermal ablation (RFA) of hepatic and renal tumors can be accompanied by non-desired tumorigenesis in residual, untreated tumor. Here, we studied the use of micelle-encapsulated siRNA to suppress IL-6-mediated local and systemic secondary effects of RFA.
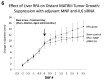
Methods: We compared standardized hepatic or renal RFA (laparotomy, 1 cm active tip at 70 ± 2 °C for 5 min) and sham procedures without and with administration of 150 nm micelle-like nanoparticle (MNP) anti-IL6 siRNA (DOPE-PEI conjugates, single IP dose 15 min post-RFA, C57Bl mouse:3.5 ug/100ml, Fisher 344 rat: 20 ug/200 ul), RFA/scrambled siRNA, and RFA/empty MNPs. Outcome measures included: local periablational cellular infiltration (α-SMA+ stellate cells), regional hepatocyte proliferation, serum/tissue IL-6 and VEGF levels at 6-72 hr, and distant tumor growth, tumor proliferation (Ki-67) and microvascular density (MVD, CD34) in subcutaneous R3230 and MATBIII breast adenocarcinoma models at 7 days.
Results: For liver RFA, adjuvant MNP anti-IL6 siRNA reduced RFA-induced increases in tissue IL-6 levels, α-SMA+ stellate cell infiltration, and regional hepatocyte proliferation to baseline (p < 0.04, all comparisons). Moreover, adjuvant MNP anti-IL6- siRNA suppressed increased distant tumor growth and Ki-67 observed in R3230 and MATBIII tumors post hepatic RFA (p<0.01). Anti-IL6 siRNA also reduced RFA-induced elevation in VEGF and tumor MVD (p < 0.01). Likewise, renal RFA-induced increases in serum IL-6 levels and distant R3230 tumor growth was suppressed with anti-IL6 siRNA (p < 0.01).
Conclusions: Adjuvant nanoparticle-encapsulated siRNA against IL-6 can be used to modulate local and regional effects of hepatic RFA to block potential unwanted pro-oncogenic effects of hepatic or renal RFA on distant tumor.
Conflict of interest statement
Figures





References
-
- Mao CP, Hung CF, Wu TC. Immunotherapeutic strategies employing RNA interference technology for the control of cancers. Journal of biomedical science. 2007. January;14(1):15–29. . - PubMed
-
- Niu J, Li XN, Qian H, Han Z. siRNA mediated the type 1 insulin-like growth factor receptor and epidermal growth factor receptor silencing induces chemosensitization of liver cancer cells. Journal of cancer research and clinical oncology. 2008. April;134(4):503–13. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

