Mechanism of brain targeting by dexibuprofen prodrugs modified with ethanolamine-related structures
- PMID: 26154870
- PMCID: PMC4671119
- DOI: 10.1038/jcbfm.2015.160
Mechanism of brain targeting by dexibuprofen prodrugs modified with ethanolamine-related structures
Abstract
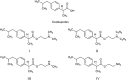
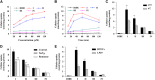
The first molecular insights into how prodrugs modified with ethanolamine-related structures target the brain were generated using an in vitro BBB model and in situ perfusion technique. Prodrugs were delivered safely and efficiently to the brain through tight interaction with the anionic membrane of brain capillary endothelial cells, observed as a shift in zeta potential, followed by uptake into the cells. Prodrugs III and IV carrying primary and secondary amine modifications appeared to enter the brain via energy-independent passive diffusion. In contrast, besides the passive diffusion, prodrugs I and II carrying tertiary amine modifications also appeared to enter via an active process that was energy and pH dependent but was independent of sodium or membrane potential. This active process involved, at least in part, the pyrilamine-sensitive H(+)/OC antiporter, for which the N,N-diethyl-based compound II showed a much lower affinity than the N,N-dimethyl-based compound I, likely due to steric hindrance. These new insights into brain-targeting mechanisms may help guide efforts to design new prodrugs.
Figures




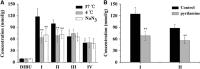
References
-
- 1Pardridge WM. Blood-brain barrier delivery. Drug Discov Today 2007; 12: 54–61. - PubMed
-
- 2Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev 2012; 64: 640–665. - PubMed
-
- 3Pardridge WM. Crossing the blood–brain barrier: are we getting it right? Drug Discov Today 2001; 6: 1–2. - PubMed
-
- 5Bonina F, Puglia C, Rimoli MG, Melisi D, Boatto G, Nieddu M et al. Glycosyl derivatives of dopamine and L-dopa as anti-Parkinson prodrugs: synthesis, pharmacological activity and in vitro stability studies. J Drug Target 2003; 11: 25–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

