Cryo-EM structure of Hepatitis C virus IRES bound to the human ribosome at 3.9-Å resolution
- PMID: 26155016
- PMCID: PMC4510694
- DOI: 10.1038/ncomms8646
Cryo-EM structure of Hepatitis C virus IRES bound to the human ribosome at 3.9-Å resolution
Abstract
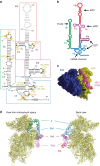
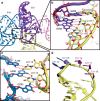
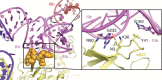
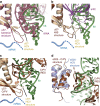
Hepatitis C virus (HCV), a widespread human pathogen, is dependent on a highly structured 5'-untranslated region of its mRNA, referred to as internal ribosome entry site (IRES), for the translation of all of its proteins. The HCV IRES initiates translation by directly binding to the small ribosomal subunit (40S), circumventing the need for many eukaryotic translation initiation factors required for mRNA scanning. Here we present the cryo-EM structure of the human 40S ribosomal subunit in complex with the HCV IRES at 3.9 Å resolution, determined by focused refinement of an 80S ribosome-HCV IRES complex. The structure reveals the molecular details of the interactions between the IRES and the 40S, showing that expansion segment 7 (ES7) of the 18S rRNA acts as a central anchor point for the HCV IRES. The structural data rationalizes previous biochemical and genetic evidence regarding the initiation mechanism of the HCV and other related IRESs.
Figures


References
-
- Gravitz L. Introduction: a smouldering public-health crisis. Nature 474, S2–S4 (2011). - PubMed
-
- Hinnebusch A. G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 83, 779–812 (2014). - PubMed
-
- Pestova T. V., Shatsky I. N., Fletcher S. P., Jackson R. J. & Hellen C. U. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev. 12, 67–83 (1998). - PMC - PubMed
-
- Otto G. A. & Puglisi J. D. The pathway of HCV IRES-mediated translation initiation. Cell 119, 369–380 (2004). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

