Cytokine signatures of human whole blood for monitoring immunosuppression
- PMID: 26155135
- PMCID: PMC4440005
- DOI: 10.5114/ceji.2014.45936
Cytokine signatures of human whole blood for monitoring immunosuppression
Abstract
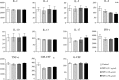
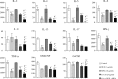
How to evaluate status of the immune system is extremely critical for clinical immunosuppressive treatment. In this study, we tested the secretion of cytokines in undiluted whole blood samples stimulated with Phorbol 12-myristate 13-acetate (PMA) and ionomycin (IONO), and compared the effects of dexamethasone (DEX), cyclosporine A (CsA) or mycophenolic acid (MPA), either alone or in combination, on cytokine profiles. The results showed that both DEX and CsA dose-dependently inhibited the production of eleven cytokines: interleukin (IL)-2, IL-4, IL-5, IL-6, IL-13, IL-17, interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF). Unexpectedly, MPA showed no obvious influences except for the mild inhibition on GM-CSF production. In combination treatment, cytokine profiles reflect not only the synergistic effects among drugs, but also the specific effect of the individual drug. Thus, the effects of different immunosuppressants could be reflected through their specific cytokine signatures, which can be applied to maximize immunosuppressive effects, while to minimize risk of infections and help physicians to reasonably apply immunosuppressants.
Keywords: autoimmune immune diseases; cytokine; immune status; immunosuppression; infection; rejection.
Figures


Similar articles
-
Evaluating the effects of immunosuppressants on human immunity using cytokine profiles of whole blood.Cytokine. 2009 Feb;45(2):141-7. doi: 10.1016/j.cyto.2008.12.003. Epub 2009 Jan 9. Cytokine. 2009. PMID: 19138532
-
Effect of mycophenolate mofetil (RS-61443) on cytokine production: inhibition of superantigen-induced cytokines.Immunopharmacology. 1993 Jul-Aug;26(1):11-20. doi: 10.1016/0162-3109(93)90062-u. Immunopharmacology. 1993. PMID: 8407281
-
Synergism of glucocorticoids with granulocyte macrophage colony stimulating factor (GM-CSF) but not interferon gamma (IFN-gamma) or interleukin-4 (IL-4) on induction of HLA class II expression on human monocytes.Cytokine. 1992 Jul;4(4):287-97. doi: 10.1016/1043-4666(92)90069-4. Cytokine. 1992. PMID: 1515553
-
Regulation of bronchoalveolar macrophage proinflammatory cytokine production by dexamethasone and granulocyte-macrophage colony-stimulating factor after stimulation by Aspergillus conidia or lipopolysaccharide.Cytokine. 2002 Jul 7;19(1):14-20. doi: 10.1006/cyto.2002.1049. Cytokine. 2002. PMID: 12200108
-
The role of monocyte-derived hemopoietic growth factors in the regulation of myeloproliferation in juvenile chronic myelogenous leukemia.Exp Hematol. 1991 Nov;19(10):1017-24. Exp Hematol. 1991. PMID: 1915702
Cited by
-
Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4‒JAK‒STAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis.J Clin Med. 2020 Nov 20;9(11):3741. doi: 10.3390/jcm9113741. J Clin Med. 2020. PMID: 33233866 Free PMC article. Review.
-
Mycophenolic Acid Synergizing with Lipopolysaccharide to Induce Interleukin-1β Release via Activation of Caspase-1.Chin Med J (Engl). 2018 Jul 5;131(13):1533-1540. doi: 10.4103/0366-6999.235116. Chin Med J (Engl). 2018. PMID: 29941706 Free PMC article.
-
Safety and outcome of elective synthetic mesh repair for incisional ventral hernias in immunosuppressed patients - a retrospective propensity-score-matched analysis.Hernia. 2025 Feb 24;29(1):106. doi: 10.1007/s10029-025-03273-3. Hernia. 2025. PMID: 39992451 Free PMC article.
-
T-lymphocyte in ANCA-associated vasculitis: what do we know? A pathophysiological and therapeutic approach.Clin Kidney J. 2019 Apr 19;12(4):503-511. doi: 10.1093/ckj/sfz029. eCollection 2019 Aug. Clin Kidney J. 2019. PMID: 31384441 Free PMC article.
-
Evidence for a More Disrupted Immune-Endocrine Relation and Cortisol Immunologic Influences in the Context of Tuberculosis and Type 2 Diabetes Comorbidity.Front Endocrinol (Lausanne). 2020 Mar 20;11:126. doi: 10.3389/fendo.2020.00126. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32265833 Free PMC article.
References
-
- Ashton-Chess J, Giral M, Soulillou JP, Brouard S. Can immune monitoring help to minimize immunosuppression in kidney transplantation? Transpl Int. 2009;22:110–119. - PubMed
-
- Chapman J. Addressing the challenges for improving long-term outcomes in renal transplantation. Transplant Proc. 2008;40(10 Suppl):S2–4. - PubMed
-
- Weimar W, Rischen-Vos J, de Kuiper P, et al. Tapering immunosuppression in recipients of living donor kidney transplants. Nephrol Dial Transplant. 2004;19(Suppl 4):iv61–63. - PubMed
-
- Kim DH, Kim JG, Sohn SK, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br J Haematol. 2004;125:217–224. - PubMed
-
- Draskovic-Pavlovic B, Hrvacevic R, Ignjatovic Lj, et al. Importance of determining the absolute CD3+ lymphocyte count during induction therapy with antithymocyte globulin in kidney transplantation patients. Vojnosanit Pregl. 2000;57:285–290. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
