Understanding high endothelial venules: Lessons for cancer immunology
- PMID: 26155419
- PMCID: PMC4485764
- DOI: 10.1080/2162402X.2015.1008791
Understanding high endothelial venules: Lessons for cancer immunology
Abstract
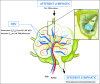
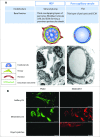
High endothelial venules (HEVs) are blood vessels especially adapted for lymphocyte trafficking which are normally found in secondary lymphoid organs such as lymph nodes (LN) and Peyer's patches. It has long been known that HEVs develop in non-lymphoid organs during chronic inflammation driven by autoimmunity, infection or allografts. More recently, HEVs have been observed in solid, vascularized tumors and their presence correlated with reduced tumor size and improved patient outcome. It is proposed that newly formed HEV promote antitumor immunity by recruiting naive lymphocytes into the tumor, thus allowing the local generation of cancerous tissue-destroying lymphocytes. Understanding how HEVs develop and function are therefore important to unravel their role in human cancers. In LN, HEVs develop during embryonic and early post-natal life and are actively maintained by the LN microenvironment. Systemic blockade of lymphotoxin-β receptor leads to HEV de-differentiation, but the LN components that induce HEV differentiation have remained elusive. Recent elegant studies using gene-targeted mice have demonstrated clearly that triggering the lymphotoxin-β receptor in endothelial cells (EC) induces the differentiation of HEV and that CD11c+ dendritic cells play a crucial role in this process. It will be important to determine whether lymphotoxin-β receptor-dependent signaling in EC drives the development of HEV during tumorigenesis and which cells have HEV-inducer properties. This may reveal therapeutic approaches to promote HEV neogenesis and determine the impact of newly formed HEV on tumor immunity.
Keywords: EC, endothelial cells; FRC, fibroblast reticular cells; HEC, high endothelial cells; HEV, high endothelial venules; LN, lymph nodes; LPA, lysophosphatidic acid; LT, lymphotoxin; LT-βR, lymphotoxin-β receptor; MAdCAM, mucosal cell adhesion molecule; PNAd, peripheral node addressin; SIP, sphingosine-1-phosphate; T cell homing; TLO, tertiary lymphoid organ; VE-cadherin, vascular endothelial cadherin; VEGF, vascular endothelial growth factor; dendritic cells; high endothelial venules; lymphotoxin-β receptor; tumor immunotherapy.
Figures





References
-
- Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 2012; 12:762-73; PMID:23018291; http://dx.doi.org/10.1038/nri3298 - DOI - PubMed
-
- Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol 2013; 13:309-20; PMID:23598650; http://dx.doi.org/10.1038/nri3442 - DOI - PubMed
-
- Gowans JL, Knight EJ. The route of recirculation of lymphocytes in the rat. Proc R Soc Series B 1964; 159:257-82; http://dx.doi.org/10.1098/rspb.1964.0001 - DOI - PubMed
-
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006; 25:989-1001; PMID:17112751; http://dx.doi.org/10.1016/j.immuni.2006.10.011 - DOI - PMC - PubMed
-
- Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol 2009; 9:618-29; PMID:19644499; http://dx.doi.org/10.1038/nri2588 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
