Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression
- PMID: 26155422
- PMCID: PMC4485824
- DOI: 10.1080/2162402X.2015.1008824
Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression
Abstract
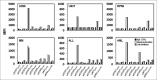
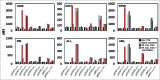
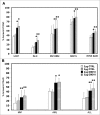
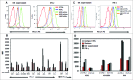
Inhibition of JAK1 or JAK2 in human tumor cells was previously shown to increase susceptibility of these cells to NK cell lysis. In the present study, we examined the cellular mechanisms that mediate this effect in hematopoietic tumor cell lines and primary tumor cells. Incubation of tumor cells with supernatant from activated NK cells or interferon-gamma (IFNγ)-induced activation of pSTAT1 and increased expression of PD-L1 without altering expression of other activating or inhibitory NK cell ligands. These functional effects were blocked by chemical JAK inhibition or shRNAs targeting JAK1, JAK2 or STAT1. Inhibition of IFNγ signaling also prevented the upregulation of PD-L1 and blocking PD-L1 resulted in increased tumor lysis by NK cells. These results show that NK cell activation and secretion of IFNγ results in activation of JAK1, JAK2 and STAT1 in tumor cells, resulting in rapid up-regulation of PD-L1 expression. Increased expression of PD-L1 results in increased resistance to NK cell lysis. Blockade of JAK pathway activation prevents increased PD-L1 expression resulting in increased susceptibility of tumor cells to NK cell activity. These observations suggest that JAK pathway inhibitors as well as PD-1 and PD-L1 antibodies may work synergistically with other immune therapies by preventing IFN-induced inhibition of NK cell-mediated tumor cell lysis.
Keywords: ADCC, Antibody dependent cellular cytotoxicity; AKT, Ak strain transforming; APC, Allophycocyanin; CTRL, Control; DMSO, Dimethyl sulfoxide; ERK, extracellular-signal-regulated kinases; IFNγ; JAK1/JAK2; MACS, Magnetic cell separation; MAPK, Mitogen-activated protein kinases; NK cells; PD-1/PD-L1; RAS, Rat sarcoma; STAT, signal transducer and activator of transcription.
Figures


References
-
- Caligiuri MA. Human natural killer cells. Blood 2008; 112:461-9; PMID:; http://dx.doi.org/10.1182/blood-2007-09-077438 - DOI - PMC - PubMed
-
- Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell 2010; 142:847-56; PMID:; http://dx.doi.org/10.1016/j.cell.2010.08.031 - DOI - PMC - PubMed
-
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:; http://dx.doi.org/10.1126/science.1198687 - DOI - PMC - PubMed
-
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9:495-502; PMID:; http://dx.doi.org/10.1038/ni1581 - DOI - PMC - PubMed
-
- Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood 1990; 76:2421-38; PMID: - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
