Test characteristics and potential impact of the urine LAM lateral flow assay in HIV-infected outpatients under investigation for TB and able to self-expectorate sputum for diagnostic testing
- PMID: 26156025
- PMCID: PMC4495934
- DOI: 10.1186/s12879-015-0967-z
Test characteristics and potential impact of the urine LAM lateral flow assay in HIV-infected outpatients under investigation for TB and able to self-expectorate sputum for diagnostic testing
Abstract
Background: The commercially available urine LAM strip test, a point-of-care tuberculosis (TB) assay, requires evaluation in a primary care setting where it is most needed. There is currently inadequate data to guide implementation in TB and HIV-endemic settings.
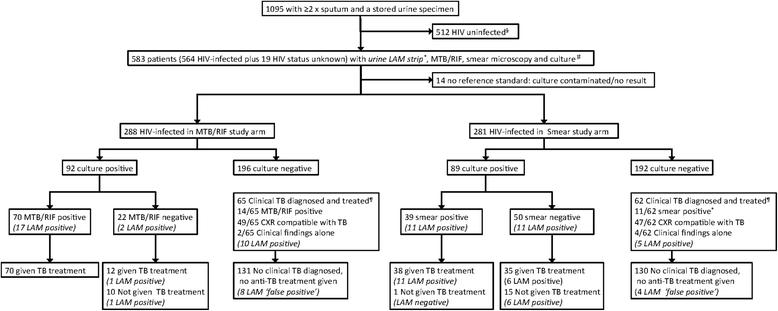
Methods: Adult HIV-infected outpatients with suspected pulmonary TB able to self-expectorate sputum from four primary clinics in South Africa, Zambia and Tanzania underwent diagnostic evaluation [sputum smear microscopy, Xpert-MTB/RIF, and culture (reference standard)] as part of a prospective parent study. Urine LAM testing (grade-2 cut-point) was performed on archived samples. Performance characteristics of LAM alone or in combination with sputum-based diagnostics were evaluated. Potential impact on 2 and 6-month morbidity (TBscore), patient dropout rates, and prognosis (death/ loss to follow-up) were evaluated.
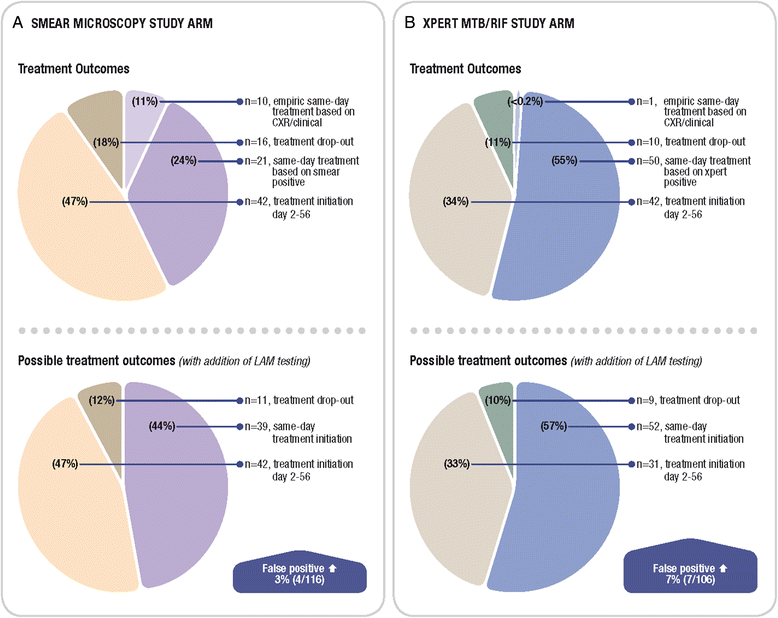
Results: Among 583 participants with suspected TB that were HIV-infected or refused testing, the overall LAM sensitivity (95 % CI; n/N) and in the CD4 ≤ 100 cells/mm(3) sub-group was 22.7 % (16.6-28.7; 41/181) and 30.4 % (17.1-43.7; 14/46), respectively. Overall specificity was 93.0 % (90.5-95.6; 361/388). Amongst culture-positive TB cases, adjunctive LAM testing did not improve the sensitivity of either sputum Xpert-MTB/RIF [78.2 % (69.8-86.7; 72/92) versus 76.1 % (67.4-84.8; 70/92), p = 0.7] or smear-microscopy [56.2 % (45.9-66.5; 50/89) versus 43.8 % (33.5-54.1; 39/89), p = 0.1). Clinic-based LAM, as an adjunct to either smear microscopy or Xpert MTB/RIF same-day testing, would neither have decreased patient dropout, nor increased same-day treatment initiation in this clinical setting where same-day chest radiography was available. LAM positivity was associated with 6-month lost-to-follow-up/death (AOR 4.4; p = 0.002) but not TBscore (at baseline or change in TBscore 2-months post-treatment) (p = 0.17).
Conclusions: In African HIV-TB co-infected outpatients able to self-expectorate sputum LAM had limited sensitivity even at low CD4 counts, and offered no significant incremental diagnostic yield over Xpert-MTB/RIF or smear microscopy. In primary care clinics with chest radiography and where empiric TB treatment is common, LAM seems unlikely to improve rates of same-day treatment initiation and patient dropout, however, the ability of LAM to identify patients at high risk of death or lost-to-follow-up may offer important prognostic value.
Figures
References
-
- WHO . WHO Report 2013: Global tuberculosis control. Geneva: World Heath Organisation; 2013.
-
- Dowdy DW, Chaisson RE, Moulton LH, Dorman SE. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: a mathematical model. AIDS. 2006;20(5):751–62. - PubMed
-
- Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383(9915):424–35. doi: 10.1016/S0140-6736(13)62073-5. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

