Cell-cell signalling in sexual chemotaxis: a basis for gametic differentiation, mating types and sexes
- PMID: 26156301
- PMCID: PMC4535405
- DOI: 10.1098/rsif.2015.0342
Cell-cell signalling in sexual chemotaxis: a basis for gametic differentiation, mating types and sexes
Abstract
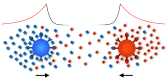
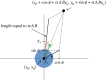
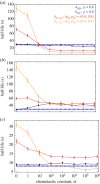
While sex requires two parents, there is no obvious need for them to be differentiated into distinct mating types or sexes. Yet this is the predominate state of nature. Here, we argue that mating types could play a decisive role because they prevent the apparent inevitability of self-stimulation during sexual signalling. We rigorously assess this hypothesis by developing a model for signaller-detector dynamics based on chemical diffusion, chemotaxis and cell movement. Our model examines the conditions under which chemotaxis improves partner finding. Varying parameter values within ranges typical of protists and their environments, we show that simultaneous secretion and detection of a single chemoattractant can cause a multifold movement impediment and severely hinder mate finding. Mutually exclusive roles result in faster pair formation, even when cells conferring the same roles cannot pair up. This arrangement also allows the separate mating types to optimize their signalling or detecting roles, which is effectively impossible for cells that are both secretors and detectors. Our findings suggest that asymmetric roles in sexual chemotaxis (and possibly other forms of sexual signalling) are crucial, even without morphological differences, and may underlie the evolution of gametic differentiation among both mating types and sexes.
Keywords: chemotaxis; mating types; sexes; signalling.
© 2015 The Authors.
Figures




Similar articles
-
Gamete signalling underlies the evolution of mating types and their number.Philos Trans R Soc Lond B Biol Sci. 2016 Oct 19;371(1706):20150531. doi: 10.1098/rstb.2015.0531. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27619695 Free PMC article.
-
Strong-coupling dynamics of a multicellular chemotactic system.Phys Rev Lett. 2005 Sep 16;95(12):128103. doi: 10.1103/PhysRevLett.95.128103. Epub 2005 Sep 15. Phys Rev Lett. 2005. PMID: 16197116
-
The effect of chemotaxis on T-cell regulatory dynamics.J Math Biol. 2023 Nov 10;87(6):84. doi: 10.1007/s00285-023-02017-0. J Math Biol. 2023. PMID: 37947884
-
Follow the leader.Dev Cell. 2003 Mar;4(3):291-3. doi: 10.1016/s1534-5807(03)00064-9. Dev Cell. 2003. PMID: 12636911 Review.
-
Sexual selection in fungi.J Evol Biol. 2012 Dec;25(12):2397-411. doi: 10.1111/jeb.12017. J Evol Biol. 2012. PMID: 23163326 Review.
Cited by
-
The evolution of mating type switching.Evolution. 2016 Jul;70(7):1569-81. doi: 10.1111/evo.12959. Epub 2016 Jun 17. Evolution. 2016. PMID: 27271362 Free PMC article.
-
Genome analyses of the new model protist Euplotes vannus focusing on genome rearrangement and resistance to environmental stressors.Mol Ecol Resour. 2019 Sep;19(5):1292-1308. doi: 10.1111/1755-0998.13023. Epub 2019 Jun 6. Mol Ecol Resour. 2019. PMID: 30985983 Free PMC article.
-
Evolution of asymmetric gamete signaling and suppressed recombination at the mating type locus.Elife. 2019 Aug 29;8:e48239. doi: 10.7554/eLife.48239. Elife. 2019. PMID: 31464685 Free PMC article.
-
Invasion and Extinction Dynamics of Mating Types Under Facultative Sexual Reproduction.Genetics. 2019 Oct;213(2):567-580. doi: 10.1534/genetics.119.302306. Epub 2019 Aug 7. Genetics. 2019. PMID: 31391266 Free PMC article.
-
Gamete signalling underlies the evolution of mating types and their number.Philos Trans R Soc Lond B Biol Sci. 2016 Oct 19;371(1706):20150531. doi: 10.1098/rstb.2015.0531. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27619695 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

