The Breast Cancer to Bone (B2B) Metastases Research Program: a multi-disciplinary investigation of bone metastases from breast cancer
- PMID: 26156521
- PMCID: PMC4496930
- DOI: 10.1186/s12885-015-1528-y
The Breast Cancer to Bone (B2B) Metastases Research Program: a multi-disciplinary investigation of bone metastases from breast cancer
Abstract
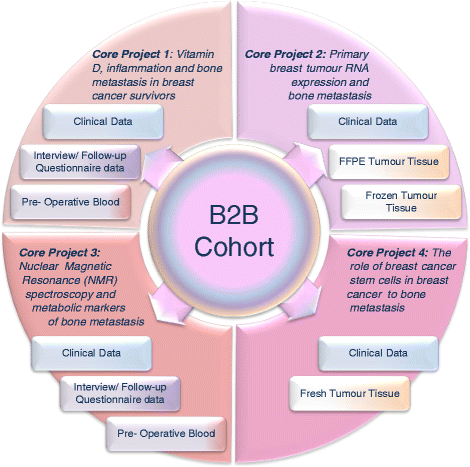
Background: Bone is the most common site of breast cancer distant metastasis, affecting 50-70 % of patients who develop metastatic disease. Despite decades of informative research, the effective prevention, prediction and treatment of these lesions remains elusive. The Breast Cancer to Bone (B2B) Metastases Research Program consists of a prospective cohort of incident breast cancer patients and four sub-projects that are investigating priority areas in breast cancer bone metastases. These include the impact of lifestyle factors and inflammation on risk of bone metastases, the gene expression features of the primary tumour, the potential role for metabolomics in early detection of bone metastatic disease and the signalling pathways that drive the metastatic lesions in the bone.
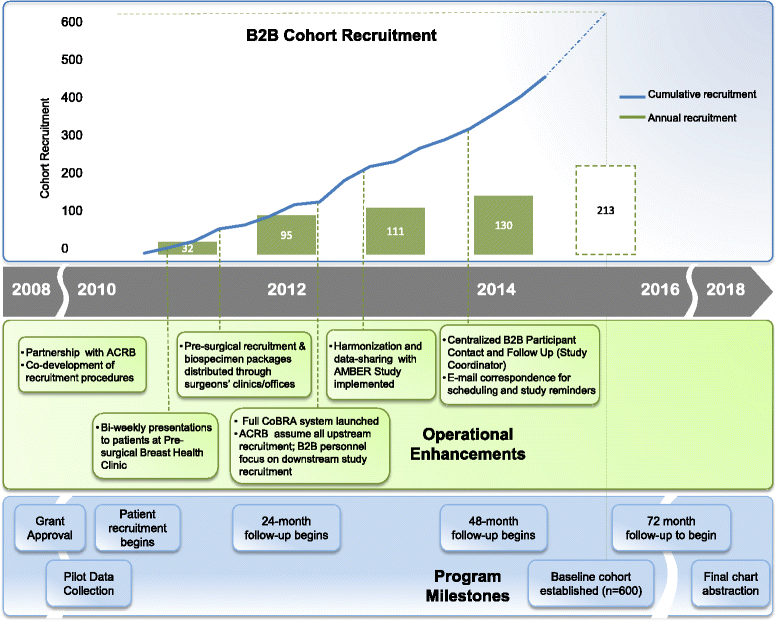
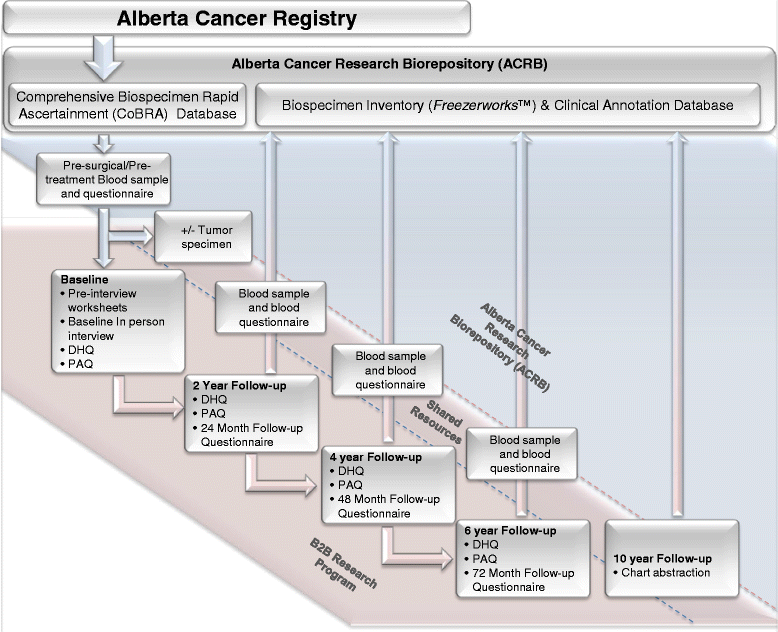
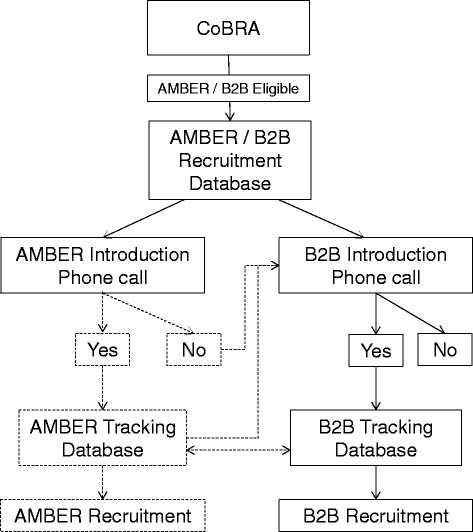
Methods/design: The B2B Research Program is enrolling a prospective cohort of 600 newly diagnosed, incident, stage I-IIIc breast cancer survivors in Alberta, Canada over a five year period. At baseline, pre-treatment/surgery blood samples are collected and detailed epidemiologic data is collected by in-person interview and self-administered questionnaires. Additional self-administered questionnaires and blood samples are completed at specified follow-up intervals (24, 48 and 72 months). Vital status is obtained prior to each follow-up through record linkages with the Alberta Cancer Registry. Recurrences are identified through medical chart abstractions. Each of the four projects applies specific methods and analyses to assess the impact of serum vitamin D and cytokine concentrations, tumour transcript and protein expression, serum metabolomic profiles and in vitro cell signalling on breast cancer bone metastases.
Discussion: The B2B Research Program will address key issues in breast cancer bone metastases including the association between lifestyle factors (particularly a comprehensive assessment of vitamin D status) inflammation and bone metastases, the significance or primary tumour gene expression in tissue tropism, the potential of metabolomic profiles for risk assessment and early detection and the signalling pathways controlling the metastatic tumour microenvironment. There is substantial synergy between the four projects and it is hoped that this integrated program of research will advance our understanding of key aspects of bone metastases from breast cancer to improve the prevention, prediction, detection, and treatment of these lesions.
Figures
Similar articles
-
The Breast to Bone (B2B) Cohort Study to Prevent, Detect and Improve Treatment of Metastatic Disease: Baseline Assessment, Description and Progress.Int J Environ Res Public Health. 2025 Feb 8;22(2):242. doi: 10.3390/ijerph22020242. Int J Environ Res Public Health. 2025. PMID: 40003468 Free PMC article.
-
A novel gene expression signature for bone metastasis in breast carcinomas.Breast Cancer Res Treat. 2016 Apr;156(2):249-59. doi: 10.1007/s10549-016-3741-z. Epub 2016 Mar 10. Breast Cancer Res Treat. 2016. PMID: 26965286 Free PMC article.
-
Metastatic progression of breast cancer: insights from 50 years of autopsies.J Pathol. 2014 Jan;232(1):23-31. doi: 10.1002/path.4288. J Pathol. 2014. PMID: 24122263 Free PMC article.
-
Molecular interactions between breast cancer cells and the bone microenvironment drive skeletal metastases.Cancer Metastasis Rev. 2006 Dec;25(4):621-33. doi: 10.1007/s10555-006-9023-1. Cancer Metastasis Rev. 2006. PMID: 17165131 Review.
-
Expression of bone matrix proteins in human breast cancer: potential roles in microcalcification formation and in the genesis of bone metastases.Bull Cancer. 1997 Jan;84(1):17-24. Bull Cancer. 1997. PMID: 9180854 Review.
Cited by
-
Plasma hPG80 (Circulating Progastrin) as a Novel Prognostic Biomarker for early-stage breast cancer in a breast cancer cohort.BMC Cancer. 2023 Apr 4;23(1):305. doi: 10.1186/s12885-023-10729-1. BMC Cancer. 2023. PMID: 37016331 Free PMC article.
-
Anterior Gradient 2 is a Significant Prognostic Biomarker in Bone Metastasis of Breast Cancer.Pathol Oncol Res. 2022 Nov 3;28:1610538. doi: 10.3389/pore.2022.1610538. eCollection 2022. Pathol Oncol Res. 2022. PMID: 36405393 Free PMC article.
-
The Role of Prolactin in Bone Metastasis and Breast Cancer Cell-Mediated Osteoclast Differentiation.J Natl Cancer Inst. 2015 Nov 19;108(3):djv338. doi: 10.1093/jnci/djv338. Print 2016 Mar. J Natl Cancer Inst. 2015. PMID: 26586670 Free PMC article.
-
Prospective comparison of the diagnostic accuracy of 18F-FDG PET/MRI, MRI, CT, and bone scintigraphy for the detection of bone metastases in the initial staging of primary breast cancer patients.Eur Radiol. 2021 Nov;31(11):8714-8724. doi: 10.1007/s00330-021-07956-0. Epub 2021 Apr 28. Eur Radiol. 2021. PMID: 33912991 Free PMC article.
-
Androgen receptor variant-7 regulation by tenascin-c induced src activation.Cell Commun Signal. 2022 Aug 10;20(1):119. doi: 10.1186/s12964-022-00925-0. Cell Commun Signal. 2022. PMID: 35948987 Free PMC article.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. - PubMed
-
- Canadian Cancer Statistics 2014 [http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20...]
-
- Glendenning J, Cook G. Imaging breast cancer bone metastases: current status and future directions. Semin Nucl Med. 2013;43(4):317–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

