Kallistatin inhibits TGF-β-induced endothelial-mesenchymal transition by differential regulation of microRNA-21 and eNOS expression
- PMID: 26156753
- PMCID: PMC4560618
- DOI: 10.1016/j.yexcr.2015.06.021
Kallistatin inhibits TGF-β-induced endothelial-mesenchymal transition by differential regulation of microRNA-21 and eNOS expression
Abstract
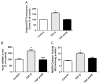
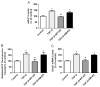
Kallistatin, an endogenous protein, consists of two structural elements: active site and heparin-binding domain. Kallistatin exerts beneficial effects on fibrosis by suppressing transforming growth factor (TGF)-β synthesis in animal models. TGF-β is the most potent inducer of endothelial-mesenchymal transition (EndMT), which contributes to fibrosis and cancer. MicroRNA (miR)-21 is an important player in organ fibrosis and tumor invasion. Here we investigated the potential role of kallistatin in EndMT via modulation of miR-21 in endothelial cells. Human kallistatin treatment blocked TGF-β-induced EndMT, as evidenced by morphological changes as well as increased endothelial and reduced mesenchymal marker expression. Kallistatin also inhibited TGF-β-mediated reactive oxygen species (ROS) formation and NADPH oxidase expression and activity. Moreover, kallistatin antagonized TGF-β-induced miR-21 and Snail1 synthesis, Akt phosphorylation, NF-κB activation, and matrix metalloproteinase 2 (MMP2) synthesis and activation. Kallistatin via its heparin-binding site blocked TGF-β-induced miR-21, Snail1 expression, and ROS formation, as wild-type kallistatin, but not heparin-binding site mutant kallistatin, exerted the effect. Conversely, kallistatin through its active site stimulated the synthesis of endothelial nitric oxide synthase (eNOS), sirtuin 1 (Sirt1) and forkhead box O1 (FoxO1); however, these effects were blocked by genistein, a tyrosine kinase inhibitor. This is the first study to demonstrate that kallistatin's heparin-binding site is crucial for preventing TGF-β-induced miR-21 and oxidative stress, while its active site is key for stimulating the expression of antioxidant genes via interaction with an endothelial surface tyrosine kinase. These findings reveal novel mechanisms of kallistatin in protection against fibrosis and cancer by suppressing EndMT.
Keywords: EndMT; Kallistatin; Oxidative stress; TGF-β; eNOS; miR-21.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Lin F, Wang N, Zhang TC. The role of endothelial–mesenchymal transition in development and pathological process. IUBMB Life. 2012;64:717–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

