fMRI Analysis-by-Synthesis Reveals a Dorsal Hierarchy That Extracts Surface Slant
- PMID: 26156985
- PMCID: PMC4495240
- DOI: 10.1523/JNEUROSCI.1255-15.2015
fMRI Analysis-by-Synthesis Reveals a Dorsal Hierarchy That Extracts Surface Slant
Abstract
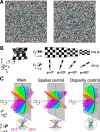
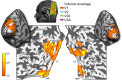
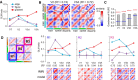
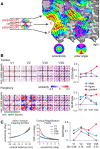
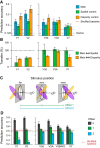
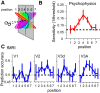
The brain's skill in estimating the 3-D orientation of viewed surfaces supports a range of behaviors, from placing an object on a nearby table, to planning the best route when hill walking. This ability relies on integrating depth signals across extensive regions of space that exceed the receptive fields of early sensory neurons. Although hierarchical selection and pooling is central to understanding of the ventral visual pathway, the successive operations in the dorsal stream are poorly understood. Here we use computational modeling of human fMRI signals to probe the computations that extract 3-D surface orientation from binocular disparity. To understand how representations evolve across the hierarchy, we developed an inference approach using a series of generative models to explain the empirical fMRI data in different cortical areas. Specifically, we simulated the responses of candidate visual processing algorithms and tested how well they explained fMRI responses. Thereby we demonstrate a hierarchical refinement of visual representations moving from the representation of edges and figure-ground segmentation (V1, V2) to spatially extensive disparity gradients in V3A. We show that responses in V3A are little affected by low-level image covariates, and have a partial tolerance to the overall depth position. Finally, we show that responses in V3A parallel perceptual judgments of slant. This reveals a relatively short computational hierarchy that captures key information about the 3-D structure of nearby surfaces, and more generally demonstrates an analysis approach that may be of merit in a diverse range of brain imaging domains.
Keywords: 3-D vision; binocular disparity; fMRI; slant.
Copyright © 2015 Ban and Welchman.
Figures



Similar articles
-
Perceptual integration for qualitatively different 3-D cues in the human brain.J Cogn Neurosci. 2013 Sep;25(9):1527-41. doi: 10.1162/jocn_a_00417. Epub 2013 May 6. J Cogn Neurosci. 2013. PMID: 23647559 Free PMC article.
-
7 tesla FMRI reveals systematic functional organization for binocular disparity in dorsal visual cortex.J Neurosci. 2015 Feb 18;35(7):3056-72. doi: 10.1523/JNEUROSCI.3047-14.2015. J Neurosci. 2015. PMID: 25698743 Free PMC article.
-
Ultra-High-Field Neuroimaging Reveals Fine-Scale Processing for 3D Perception.J Neurosci. 2021 Oct 6;41(40):8362-8374. doi: 10.1523/JNEUROSCI.0065-21.2021. Epub 2021 Aug 19. J Neurosci. 2021. PMID: 34413206 Free PMC article.
-
The Human Brain in Depth: How We See in 3D.Annu Rev Vis Sci. 2016 Oct 14;2:345-376. doi: 10.1146/annurev-vision-111815-114605. Epub 2016 Jul 22. Annu Rev Vis Sci. 2016. PMID: 28532360 Review.
-
How does binocular rivalry emerge from cortical mechanisms of 3-D vision?Vision Res. 2008 Sep;48(21):2232-50. doi: 10.1016/j.visres.2008.06.024. Epub 2008 Aug 13. Vision Res. 2008. PMID: 18640145 Review.
Cited by
-
Unique Neural Activity Patterns Among Lower Order Cortices and Shared Patterns Among Higher Order Cortices During Processing of Similar Shapes With Different Stimulus Types.Iperception. 2021 May 26;12(3):20416695211018222. doi: 10.1177/20416695211018222. eCollection 2021 May-Jun. Iperception. 2021. PMID: 34104383 Free PMC article.
-
fMRI Activity in Posterior Parietal Cortex Relates to the Perceptual Use of Binocular Disparity for Both Signal-In-Noise and Feature Difference Tasks.PLoS One. 2015 Nov 3;10(11):e0140696. doi: 10.1371/journal.pone.0140696. eCollection 2015. PLoS One. 2015. PMID: 26529314 Free PMC article.
-
Identifying Cortical Substrates Underlying the Phenomenology of Stereopsis and Realness: A Pilot fMRI Study.Front Neurosci. 2019 Jul 11;13:646. doi: 10.3389/fnins.2019.00646. eCollection 2019. Front Neurosci. 2019. PMID: 31354404 Free PMC article.
-
Choice-Related Activity during Visual Slant Discrimination in Macaque CIP But Not V3A.eNeuro. 2019 Mar 26;6(2):ENEURO.0248-18.2019. doi: 10.1523/ENEURO.0248-18.2019. eCollection 2019 Mar-Apr. eNeuro. 2019. PMID: 30923736 Free PMC article.
-
A dataset of stereoscopic images and ground-truth disparity mimicking human fixations in peripersonal space.Sci Data. 2017 Mar 28;4:170034. doi: 10.1038/sdata.2017.34. Sci Data. 2017. PMID: 28350382 Free PMC article.
References
-
- Backus BT, Fleet DJ, Parker AJ, Heeger DJ. Human cortical activity correlates with stereoscopic depth perception. J Neurophysiol. 2001;86:2054–2068. - PubMed
-
- Chandrasekaran C, Canon V, Dahmen JC, Kourtzi Z, Welchman AE. Neural correlates of disparity-defined shape discrimination in the human brain. J Neurophysiol. 2007;97:1553–1565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
