Neuronal Differentiation in Schwann Cell Lineage Underlies Postnatal Neurogenesis in the Enteric Nervous System
- PMID: 26156989
- PMCID: PMC6605410
- DOI: 10.1523/JNEUROSCI.1239-15.2015
Neuronal Differentiation in Schwann Cell Lineage Underlies Postnatal Neurogenesis in the Enteric Nervous System
Abstract
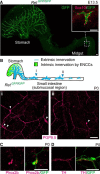
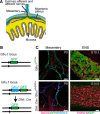
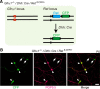
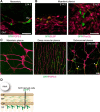
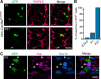
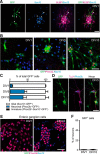
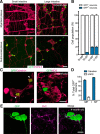
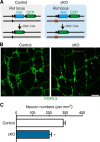
Elucidation of the cellular identity of neuronal precursors provides mechanistic insights into the development and pathophysiology of the nervous system. In the enteric nervous system (ENS), neurogenesis persists from midgestation to the postnatal period. Cellular mechanism underlying the long-term neurogenesis in the ENS has remained unclear. Using genetic fate mapping in mice, we show here that a subset of Schwann cell precursors (SCPs), which invades the gut alongside the extrinsic nerves, adopts a neuronal fate in the postnatal period and contributes to the ENS. We found SCP-derived neurogenesis in the submucosal region of the small intestine in the absence of vagal neural crest-derived ENS precursors. Under physiological conditions, SCPs comprised up to 20% of enteric neurons in the large intestine and gave rise mainly to restricted neuronal subtypes, calretinin-expressing neurons. Genetic ablation of Ret, the signaling receptor for glial cell line-derived neurotrophic factor, in SCPs caused colonic oligoganglionosis, indicating that SCP-derived neurogenesis is essential to ENS integrity. Identification of Schwann cells as a physiological neurogenic source provides novel insight into the development and disorders of neural crest-derived tissues.
Significance statement: Elucidating the cellular identity of neuronal precursors provides novel insights into development and function of the nervous system. The enteric nervous system (ENS) is innervated richly by extrinsic nerve fibers, but little is known about the significance of extrinsic innervation to the structural integrity of the ENS. This report reveals that a subset of Schwann cell precursors (SCPs), which invades the gut alongside the extrinsic nerves, adopts a neuronal fate and differentiates into specific neuronal subtypes. SCP-specific ablation of the Ret gene leads to colonic oligoganglionosis, demonstrating a crucial role of SCP-derived neurogenesis in ENS development. Cross-lineage differentiation capacity in SCPs suggests their potential involvement in the development and pathology of a wide variety of neural crest-derived cell types.
Keywords: RET; Schwann cells; enteric nervous system; neural crest cells; neurogenesis; oligoganglionosis.
Copyright © 2015 the authors 0270-6474/15/359879-10$15.00/0.
Figures

Similar articles
-
Enhanced enteric neurogenesis by Schwann cell precursors in mouse models of Hirschsprung disease.Glia. 2021 Nov;69(11):2575-2590. doi: 10.1002/glia.24059. Epub 2021 Jul 17. Glia. 2021. PMID: 34272903
-
The timing and location of glial cell line-derived neurotrophic factor expression determine enteric nervous system structure and function.J Neurosci. 2010 Jan 27;30(4):1523-38. doi: 10.1523/JNEUROSCI.3861-09.2010. J Neurosci. 2010. PMID: 20107080 Free PMC article.
-
Endoderm-derived Sonic hedgehog and mesoderm Hand2 expression are required for enteric nervous system development in zebrafish.Dev Biol. 2008 Jun 1;318(1):52-64. doi: 10.1016/j.ydbio.2008.02.061. Epub 2008 Mar 20. Dev Biol. 2008. PMID: 18436202 Free PMC article.
-
Neuron-Glia Interaction in the Developing and Adult Enteric Nervous System.Cells. 2020 Dec 31;10(1):47. doi: 10.3390/cells10010047. Cells. 2020. PMID: 33396231 Free PMC article. Review.
-
Development of the intrinsic and extrinsic innervation of the gut.Dev Biol. 2016 Sep 15;417(2):158-67. doi: 10.1016/j.ydbio.2016.04.016. Epub 2016 Apr 22. Dev Biol. 2016. PMID: 27112528 Review.
Cited by
-
Cell-autonomous retinoic acid receptor signaling has stage-specific effects on mouse enteric nervous system.JCI Insight. 2021 May 24;6(10):e145854. doi: 10.1172/jci.insight.145854. JCI Insight. 2021. PMID: 33848271 Free PMC article.
-
Enteric glial biology, intercellular signalling and roles in gastrointestinal disease.Nat Rev Gastroenterol Hepatol. 2021 Aug;18(8):571-587. doi: 10.1038/s41575-021-00423-7. Epub 2021 Mar 17. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33731961 Free PMC article. Review.
-
New insights into the neurofibroma tumor cells of origin.Neurooncol Adv. 2019 Nov 10;2(Suppl 1):i13-i22. doi: 10.1093/noajnl/vdz044. eCollection 2020 Jul. Neurooncol Adv. 2019. PMID: 32642729 Free PMC article. Review.
-
Enteric glial cell diversification is influenced by spatiotemporal factors and source of neural progenitors in mice.Front Neurosci. 2024 Aug 29;18:1392703. doi: 10.3389/fnins.2024.1392703. eCollection 2024. Front Neurosci. 2024. PMID: 39268038 Free PMC article.
-
Neural tube-associated boundary caps are a major source of mural cells in the skin.Elife. 2023 Dec 14;12:e69413. doi: 10.7554/eLife.69413. Elife. 2023. PMID: 38095361 Free PMC article.
References
-
- Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Müller T, Fritz N, Beljajeva A, Mochii M, Liste I, Usoskin D, Suter U, Birchmeier C, Ernfors P. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. doi: 10.1016/j.cell.2009.07.049. - DOI - PubMed
-
- Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285:262–273. doi: 10.1002/cne.902850208. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
