Matriptase activation and shedding through PDGF-D-mediated extracellular acidosis
- PMID: 26157007
- PMCID: PMC5005266
- DOI: 10.1152/ajpcell.00043.2015
Matriptase activation and shedding through PDGF-D-mediated extracellular acidosis
Abstract
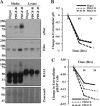
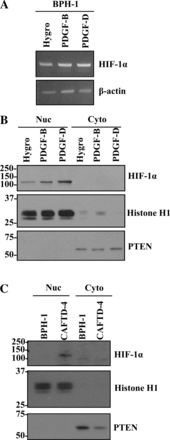
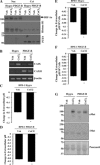
Activation of β-platelet-derived growth factor receptor (β-PDGFR) is associated with prostate cancer (PCa) progression and recurrence after prostatectomy. Analysis of the β-PDGFR ligands in PCa revealed association between PDGF-D expression and Gleason score as well as tumor stage. During the course of studying the functional consequences of PDGF ligand-specific β-PDGFR signaling in PCa, we discovered a novel function of PDGF-D for activation/shedding of the serine protease matriptase leading to cell invasion, migration, and tumorigenesis. The present study showed that PDGF-D, not PDGF-B, induces extracellular acidification, which correlates with increased matriptase activation. A cDNA microarray analysis revealed that PDGF-D/β-PDGFR signaling upregulates expression of the acidosis regulator carbonic anhydrase IX (CAIX), a classic target of the transcriptional factor hypoxia-inducible factor-1α (HIF-1α). Cellular fractionation displayed a strong HIF-1α nuclear localization in PDGF-D-expressing cells. Treatment of vector control or PDGF-B-expressing cells with the HIF-1α activator CoCl2 led to increased CAIX expression accompanied by extracellular acidosis and matriptase activation. Furthermore, the analysis of the CAFTD cell lines, variants of the BPH-1 transformation model, showed that increased PDGF-D expression is associated with enhanced HIF-1α activity, CAIX induction, cellular acidosis, and matriptase shedding. Importantly, shRNA-mediated knockdown of CAIX expression effectively reversed extracellular acidosis and matriptase activation in PDGF-D-transfected BPH-1 cells and in CAFTD variants that express endogenous PDGF-D at a high level. Taken together, these novel findings reveal a new paradigm in matriptase activation involving PDGF-D-specific signal transduction leading to extracellular acidosis.
Keywords: CAIX; HIF-1α; PDGF-D; acidosis; matriptase.
Copyright © 2016 the American Physiological Society.
Figures








Similar articles
-
A novel signaling axis of matriptase/PDGF-D/ß-PDGFR in human prostate cancer.Cancer Res. 2010 Dec 1;70(23):9631-40. doi: 10.1158/0008-5472.CAN-10-0511. Epub 2010 Nov 23. Cancer Res. 2010. PMID: 21098708 Free PMC article.
-
Differential tumorigenic potential and matriptase activation between PDGF B versus PDGF D in prostate cancer.Mol Cancer Res. 2012 Aug;10(8):1087-97. doi: 10.1158/1541-7786.MCR-12-0071. Epub 2012 Jun 11. Mol Cancer Res. 2012. PMID: 22689130 Free PMC article.
-
A novel function for platelet-derived growth factor D: induction of osteoclastic differentiation for intraosseous tumor growth.Oncogene. 2012 Oct 18;31(42):4527-35. doi: 10.1038/onc.2011.573. Epub 2011 Dec 12. Oncogene. 2012. PMID: 22158043 Free PMC article.
-
Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: A one transcription factor (HIF-1) show?Biochim Biophys Acta. 2009 Apr;1795(2):162-72. doi: 10.1016/j.bbcan.2009.01.001. Epub 2009 Jan 22. Biochim Biophys Acta. 2009. PMID: 19344680 Free PMC article. Review.
-
PDGF-C and PDGF-D signaling in vascular diseases and animal models.Mol Aspects Med. 2018 Aug;62:1-11. doi: 10.1016/j.mam.2018.01.005. Epub 2018 Feb 14. Mol Aspects Med. 2018. PMID: 29410092 Review.
Cited by
-
3-Cl-AHPC inhibits pro-HGF maturation by inducing matriptase/HAI-1 complex formation.J Cell Mol Med. 2019 Jan;23(1):155-166. doi: 10.1111/jcmm.13900. Epub 2018 Oct 28. J Cell Mol Med. 2019. PMID: 30370662 Free PMC article.
-
Targeting Breast Cancer Stem Cells.Int J Biol Sci. 2023 Jan 1;19(2):552-570. doi: 10.7150/ijbs.76187. eCollection 2023. Int J Biol Sci. 2023. PMID: 36632469 Free PMC article. Review.
-
The roles of proteases in prostate cancer.IUBMB Life. 2023 Jun;75(6):493-513. doi: 10.1002/iub.2700. Epub 2023 Jan 4. IUBMB Life. 2023. PMID: 36598826 Free PMC article. Review.
-
Unveiling the hidden risks: albumin-corrected anion gap as a superior marker for cardiovascular mortality in type 2 diabetes: insights from a nationally prospective cohort study.Front Endocrinol (Lausanne). 2024 Nov 7;15:1461047. doi: 10.3389/fendo.2024.1461047. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39574951 Free PMC article.
-
The production of recombinant platelet-derived growth factor D using the second generation modified vaccinia Ankara viral system.Protein Sci. 2022 Nov;31(11):e4468. doi: 10.1002/pro.4468. Protein Sci. 2022. PMID: 36214056 Free PMC article.
References
-
- Brahimi-Horn MC, Bellot G, Pouyssegur J. Hypoxia and energetic tumour metabolism. Curr Opin Genet Dev 21: 67–72, 2011. - PubMed
-
- Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 69: 358–368, 2009. - PubMed
-
- Dubois L, Lieuwes NG, Maresca A, Thiry A, Supuran CT, Scozzafava A, Wouters BG, Lambin P. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother Oncol 92: 423–428, 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

