Tmc gene therapy restores auditory function in deaf mice
- PMID: 26157030
- PMCID: PMC7298700
- DOI: 10.1126/scitranslmed.aab1996
Tmc gene therapy restores auditory function in deaf mice
Abstract
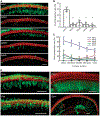
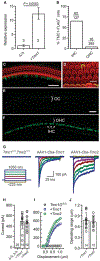
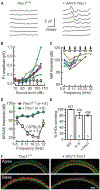
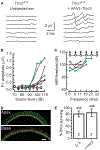
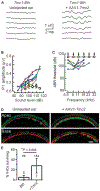
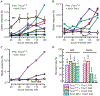
Genetic hearing loss accounts for up to 50% of prelingual deafness worldwide, yet there are no biologic treatments currently available. To investigate gene therapy as a potential biologic strategy for restoration of auditory function in patients with genetic hearing loss, we tested a gene augmentation approach in mouse models of genetic deafness. We focused on DFNB7/11 and DFNA36, which are autosomal recessive and dominant deafnesses, respectively, caused by mutations in transmembrane channel-like 1 (TMC1). Mice that carry targeted deletion of Tmc1 or a dominant Tmc1 point mutation, known as Beethoven, are good models for human DFNB7/11 and DFNA36. We screened several adeno-associated viral (AAV) serotypes and promoters and identified AAV2/1 and the chicken β-actin (Cba) promoter as an efficient combination for driving the expression of exogenous Tmc1 in inner hair cells in vivo. Exogenous Tmc1 or its closely related ortholog, Tmc2, were capable of restoring sensory transduction, auditory brainstem responses, and acoustic startle reflexes in otherwise deaf mice, suggesting that gene augmentation with Tmc1 or Tmc2 is well suited for further development as a strategy for restoration of auditory function in deaf patients who carry TMC1 mutations.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

Comment in
-
Gene therapy for deafness: How close are we?Sci Transl Med. 2015 Jul 8;7(295):295fs28. doi: 10.1126/scitranslmed.aac7545. Sci Transl Med. 2015. PMID: 26157029
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

