Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate
- PMID: 26157122
- PMCID: PMC4542370
- DOI: 10.1128/JVI.01373-15
Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate
Abstract
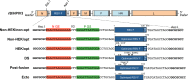
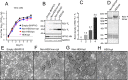
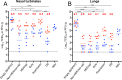
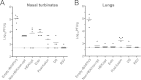
Respiratory syncytial virus (RSV) and human parainfluenza virus type 3 (HPIV3) are the first and second leading viral agents of severe respiratory tract disease in infants and young children worldwide. Vaccines are not available, and an RSV vaccine is particularly needed. A live attenuated chimeric recombinant bovine/human PIV3 (rB/HPIV3) vector expressing the RSV fusion (F) glycoprotein from an added gene has been under development as a bivalent vaccine against RSV and HPIV3. Previous clinical evaluation of this vaccine candidate suggested that increased genetic stability and immunogenicity of the RSV F insert were needed. This was investigated in the present study. RSV F expression was enhanced 5-fold by codon optimization and by modifying the amino acid sequence to be identical to that of an early passage of the original clinical isolate. This conferred a hypofusogenic phenotype that presumably reflects the original isolate. We then compared vectors expressing stabilized prefusion and postfusion versions of RSV F. In a hamster model, prefusion F induced increased quantity and quality of RSV-neutralizing serum antibodies and increased protection against wild-type (wt) RSV challenge. In contrast, a vector expressing the postfusion F was less immunogenic and protective. The genetic stability of the RSV F insert was high and was not affected by enhanced expression or the prefusion or postfusion conformation of RSV F. These studies provide an improved version of the previously well-tolerated rB/HPIV3-RSV F vaccine candidate that induces a superior RSV-neutralizing serum antibody response.
Importance: Respiratory syncytial virus (RSV) and human parainfluenza virus type 3 (HPIV3) are two major causes of pediatric pneumonia and bronchiolitis. The rB/HPIV3 vector expressing RSV F protein is a candidate bivalent live vaccine against HPIV3 and RSV. Previous clinical evaluation indicated the need to increase the immunogenicity and genetic stability of the RSV F insert. Here, we increased RSV F expression by codon optimization and by modifying the RSV F amino acid sequence to conform to that of an early passage of the original isolate. This resulted in a hypofusogenic phenotype, which likely represents the original phenotype before adaptation to cell culture. We also included stabilized versions of prefusion and postfusion RSV F protein. Prefusion RSV F induced a larger quantity and higher quality of RSV-neutralizing serum antibodies and was highly protective. This provides an improved candidate for further clinical evaluation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi:10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. - PubMed
-
- Connors M, Collins PL, Firestone CY, Sotnikov AV, Waitze A, Davis AR, Hung PP, Chanock RM, Murphy BR. 1992. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia-RSV recombinants or RSV. Vaccine 10:475–484. doi:10.1016/0264-410X(92)90397-3. - DOI - PubMed
-
- Belshe RB, Newman FK, Tsai TF, Karron RA, Reisinger K, Roberton D, Marshall H, Schwartz R, King J, Henderson FW, Rodriguez W, Severs JM, Wright PF, Keyserling H, Weinberg GA, Bromberg K, Loh R, Sly P, McIntyre P, Ziegler JB, Hackell J, Deatly A, Georgiu A, Paschalis M, Wu SL, Tatem JM, Murphy B, Anderson E. 2004. Phase 2 evaluation of parainfluenza type 3 cold passage mutant 45 live attenuated vaccine in healthy children 6–18 months old. J Infect Dis 189:462–470. doi:10.1086/381184. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

