Caspase-1-mediated cytokine release from gestational tissues, placental, and cord blood
- PMID: 26157394
- PMCID: PMC4477139
- DOI: 10.3389/fphys.2015.00186
Caspase-1-mediated cytokine release from gestational tissues, placental, and cord blood
Abstract
Distinguishing between fetal and maternal inflammatory responses is necessary for understanding the immune interplay either side of the placenta. Fetal immunity reaches maturity during extrauterine life and while basic inflammatory responses afford a certain degree of protection, fetuses are vulnerable to infection. With the discovery of inflammasomes-intracellular scaffolds that facilitate the elaboration of reactions resulting in the release of mature interleukin-1β (IL-1β)-it is necessary to consider how inflammatory stimuli are processed. The purinergic P2X7 receptor located on haematopoietic cells is a key intermediary in signal transduction initiated at Toll-like receptors (TLR) terminating in release of the mature IL-1β product. We demonstrate herein that IL-1β release from fetal membranes and mononuclear cells isolated from cord, placental, and maternal blood, obtained at term, is P2X7- and caspase-1 dependent. The P2X7-dependent release of the cytokine, which was highest from choriodecidua, was attenuated by progesterone (P4), prolactin and an NFkB inhibitor. The NLRP3 inflammasome appears necessary for the processing of IL-1β in gestational tissues and leukocytes.
Keywords: IL-1β; P2X7 receptor; caspase-1; inflammasome; parturition; pregnancy.
Figures



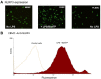
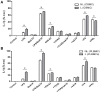

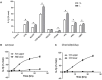
References
-
- da Fonseca E. B., Bittar R. E., Carvalho M. H., Zugaib M. (2003). Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am. J. Obstet. Gynecol. 188, 419–424. 10.1067/mob.2003.41 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

