The molecular dimension of microbial species: 3. Comparative genomics of Synechococcus strains with different light responses and in situ diel transcription patterns of associated putative ecotypes in the Mushroom Spring microbial mat
- PMID: 26157428
- PMCID: PMC4477158
- DOI: 10.3389/fmicb.2015.00604
The molecular dimension of microbial species: 3. Comparative genomics of Synechococcus strains with different light responses and in situ diel transcription patterns of associated putative ecotypes in the Mushroom Spring microbial mat
Abstract
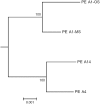
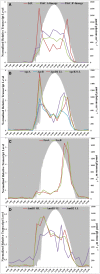
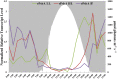
Genomes were obtained for three closely related strains of Synechococcus that are representative of putative ecotypes (PEs) that predominate at different depths in the 1 mm-thick, upper-green layer in the 60°C mat of Mushroom Spring, Yellowstone National Park, and exhibit different light adaptation and acclimation responses. The genomes were compared to the published genome of a previously obtained, closely related strain from a neighboring spring, and differences in both gene content and orthologous gene alleles between high-light-adapted and low-light-adapted strains were identified. Evidence of genetic differences that relate to adaptation to light intensity and/or quality, CO2uptake, nitrogen metabolism, organic carbon metabolism, and uptake of other nutrients were found between strains of the different putative ecotypes. In situ diel transcription patterns of genes, including genes unique to either low-light-adapted or high-light-adapted strains and different alleles of an orthologous photosystem gene, revealed that expression is fine-tuned to the different light environments experienced by ecotypes prevalent at various depths in the mat. This study suggests that strains of closely related PEs have different genomic adaptations that enable them to inhabit distinct ecological niches while living in close proximity within a microbial community.
Keywords: adaptation; comparative genomics; microbial mats; microbial species; thermophilic Synechococcus.
Figures
Similar articles
-
Acclimation of the photosynthetic apparatus to low light in a thermophilic Synechococcus sp. strain.Photosynth Res. 2022 Aug;153(1-2):21-42. doi: 10.1007/s11120-022-00918-7. Epub 2022 Apr 20. Photosynth Res. 2022. PMID: 35441927
-
The molecular dimension of microbial species: 2. Synechococcus strains representative of putative ecotypes inhabiting different depths in the Mushroom Spring microbial mat exhibit different adaptive and acclimative responses to light.Front Microbiol. 2015 Jun 29;6:626. doi: 10.3389/fmicb.2015.00626. eCollection 2015. Front Microbiol. 2015. PMID: 26175719 Free PMC article.
-
The molecular dimension of microbial species: 1. Ecological distinctions among, and homogeneity within, putative ecotypes of Synechococcus inhabiting the cyanobacterial mat of Mushroom Spring, Yellowstone National Park.Front Microbiol. 2015 Jun 22;6:590. doi: 10.3389/fmicb.2015.00590. eCollection 2015. Front Microbiol. 2015. PMID: 26157420 Free PMC article.
-
Distribution and Genomic Variation of Thermophilic Cyanobacteria in Diverse Microbial Mats at the Upper Temperature Limits of Photosynthesis.mSystems. 2022 Oct 26;7(5):e0031722. doi: 10.1128/msystems.00317-22. Epub 2022 Aug 18. mSystems. 2022. PMID: 35980085 Free PMC article.
-
Genomics, environmental genomics and the issue of microbial species.Heredity (Edinb). 2008 Feb;100(2):207-19. doi: 10.1038/sj.hdy.6801011. Epub 2007 Jun 6. Heredity (Edinb). 2008. PMID: 17551524 Review.
Cited by
-
Relationship between Microorganisms Inhabiting Alkaline Siliceous Hot Spring Mat Communities and Overflowing Water.Appl Environ Microbiol. 2020 Nov 10;86(23):e00194-20. doi: 10.1128/AEM.00194-20. Print 2020 Nov 10. Appl Environ Microbiol. 2020. PMID: 32978131 Free PMC article.
-
Idiosyncratic genome evolution of the thermophilic cyanobacterium Synechococcus at the limits of phototrophy.ISME J. 2024 Jan 8;18(1):wrae184. doi: 10.1093/ismejo/wrae184. ISME J. 2024. PMID: 39319368 Free PMC article.
-
Acclimation of the photosynthetic apparatus to low light in a thermophilic Synechococcus sp. strain.Photosynth Res. 2022 Aug;153(1-2):21-42. doi: 10.1007/s11120-022-00918-7. Epub 2022 Apr 20. Photosynth Res. 2022. PMID: 35441927
-
PhotoModPlus: A web server for photosynthetic protein prediction from genome neighborhood features.PLoS One. 2021 Mar 17;16(3):e0248682. doi: 10.1371/journal.pone.0248682. eCollection 2021. PLoS One. 2021. PMID: 33730083 Free PMC article.
-
The Dark Side of the Mushroom Spring Microbial Mat: Life in the Shadow of Chlorophototrophs. I. Microbial Diversity Based on 16S rRNA Gene Amplicons and Metagenomic Sequencing.Front Microbiol. 2016 Jun 17;7:919. doi: 10.3389/fmicb.2016.00919. eCollection 2016. Front Microbiol. 2016. PMID: 27379049 Free PMC article.
References
-
- Allewalt J. P., Bateson M. M., Revsbech N. P., Slack K., Ward D. M. (2006). Effects of temperature and light on growth of and photosynthesis by Synechococcus isolates typical of those predominating in the Octopus Spring microbial mat community of Yellowstone National Park. Appl. Environ. Microbiol. 72, 544–550. 10.1128/AEM.72.1.544-550.2006 - DOI - PMC - PubMed
-
- Arieli B., Padan E., Shahak Y. (1991). Sulfide-induced sulfide-quinone reductase activity inthylakoids of Oscillatoria limnetica. J. Biol. Chem. 266, 104–111. - PubMed
-
- Arieli B., Shahak Y., Taglicht D., Hauska G., Padan E. (1994). Purification and characterization of sulfide-quinone reductase, a novel enzyme driving anoxygenic photosynthesis in Oscillatoria limnetica. J. Biol. Chem. 269, 5705–5711. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

