High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo
- PMID: 26157437
- PMCID: PMC4477163
- DOI: 10.3389/fmicb.2015.00641
High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo
Abstract
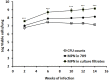
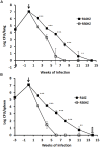
Although high-dose rifampicin holds promise for improving tuberculosis control by potentially shortening treatment duration, these effects attributed to eradication of persistent bacteria are unclear. The presence of persistent Mycobacterium tuberculosis was examined using resuscitation promoting factors (RPFs) in both in vitro hypoxia and in vivo murine tuberculosis models before and after treatment with incremental doses of rifampicin. Pharmacokinetic parameters and dose-dependent profile of rifampicin in the murine model were determined. The Cornell mouse model was used to test efficacy of high-dose rifampicin in combination with isoniazid and pyrazinamide and to measure relapse rate. There were large numbers of RPF-dependent persisters in vitro and in vivo. Stationary phase cultures were tolerant to rifampicin while higher concentrations of rifampicin eradicated plate count positive but not RPF-dependent persistent bacteria. In murine infection model, incremental doses of rifampicin exhibited a dose-dependent eradication of RPF-dependent persisters. Increasing the dose of rifampicin significantly reduced the risk of antibiotic resistance emergence. In Cornell model, mice treated with high-dose rifampicin regimen resulted in faster visceral clearance; organs were M. tuberculosis free 8 weeks post-treatment compared to 14 weeks with standard-dose rifampicin regimen. Organ sterility, plate count and RPF-dependent persister negative, was achieved. There was no disease relapse compared to the standard dose regimen (87.5%). High-dose rifampicin therapy results in eradication of RPF-dependent persisters, allowing shorter treatment duration without disease relapse. Optimizing rifampicin to its maximal efficacy with acceptable side-effect profiles will provide valuable information in human studies and can potentially improve current tuberculosis chemotherapy.
Keywords: Mycobacterium tuberculosis; mouse model; persistence; resuscitation promoting factors; rifampicin.
Figures



References
-
- Boeree M. J., Diacon A., Dawson R., Van Balen G. P., Venter A., Du Bois J., et al. (2013). What is the Right Dose of Rifampin? A Dose Escalating Study. Available at: http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2013.187.1_... - DOI
-
- de Steenwinkel J. E., Aarnoutse R. E., de Knegt G. J., Ten Kate M. T., Teulen M., Verbrugh H. A., et al. (2013). Optimization of the rifampin dosage to improve the therapeutic efficacy in tuberculosis treatment, using a murine model. Am. J. Respir. Crit. Care Med. 187 1127–1134. 10.1164/rccm.201207-1210OC - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

