Wnt/β-catenin signaling in heart regeneration
- PMID: 26157574
- PMCID: PMC4495933
- DOI: 10.1186/s13619-015-0017-8
Wnt/β-catenin signaling in heart regeneration
Abstract
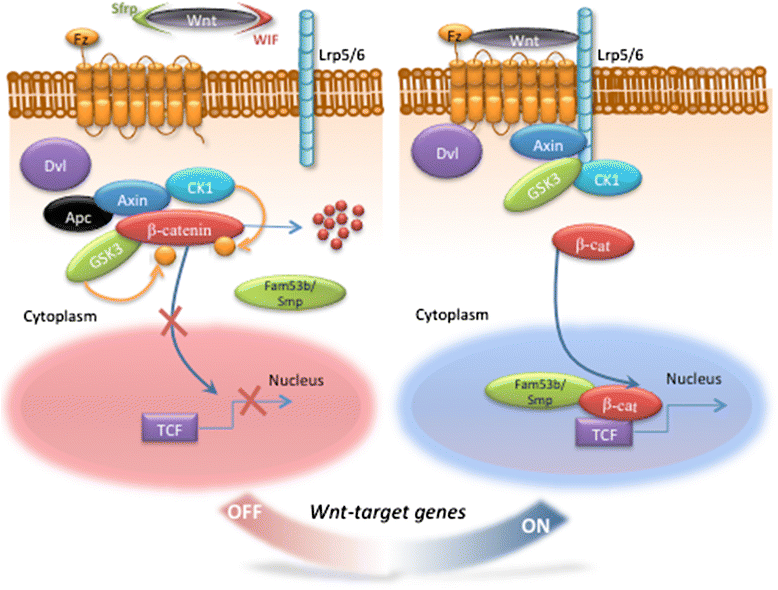
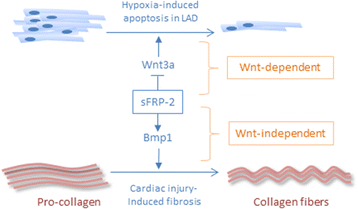
The ability to repair damaged or lost tissues varies significantly among vertebrates. The regenerative ability of the heart is clinically very relevant, because adult teleost fish and amphibians can regenerate heart tissue, but we mammals cannot. Interestingly, heart regeneration is possible in neonatal mice, but this ability is lost within 7 days after birth. In zebrafish and neonatal mice, lost cardiomyocytes are regenerated via proliferation of spared, differentiated cardiomyocytes. While some cardiomyocyte turnover occurs in adult mammals, the cardiomyocyte production rate is too low in response to injury to regenerate the heart. Instead, mammalian hearts respond to injury by remodeling of spared tissue, which includes cardiomyocyte hypertrophy. Wnt/β-catenin signaling plays important roles during vertebrate heart development, and it is re-activated in response to cardiac injury. In this review, we discuss the known functions of this signaling pathway in injured hearts, its involvement in cardiac fibrosis and hypertrophy, and potential therapeutic approaches that might promote cardiac repair after injury by modifying Wnt/β-catenin signaling. Regulation of cardiac remodeling by this signaling pathway appears to vary depending on the injury model and the exact stages that have been studied. Thus, conflicting data have been published regarding a potential role of Wnt/β-catenin pathway in promotion of fibrosis and cardiomyocyte hypertrophy. In addition, the Wnt inhibitory secreted Frizzled-related proteins (sFrps) appear to have Wnt-dependent and Wnt-independent roles in the injured heart. Thus, while the exact functions of Wnt/β-catenin pathway activity in response to injury still need to be elucidated in the non-regenerating mammalian heart, but also in regenerating lower vertebrates, manipulation of the pathway is essential for creation of therapeutically useful cardiomyocytes from stem cells in culture. Hopefully, a detailed understanding of the in vivo role of Wnt/β-catenin signaling in injured mammalian and non-mammalian hearts will also contribute to the success of current efforts towards developing regenerative therapies.
Keywords: Beta-catenin; Cardiomyocyte; Fibrosis; Heart; Hypertrophy; Regeneration; Wnt; Zebrafish; sFrp.
Figures

References
-
- Ozhan G, Weidinger G. Restoring tissue homeostasis: Wnt signaling in tissue regeneration after acute injury Wnt signaling in development and disease: molecular mechanisms and biological functions. In: Hoppler SP, Moon RT, editors. Wnt signaling in development and disease: molecular mechanisms and biological functions. Hoboken, New Jersey: Wiley-Blackwell; 2014. p. 459.
-
- Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21(11):1292–315. - PubMed
-
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–90. - PubMed
-
- Young W. Spinal cord regeneration. Cell Transplant. 2014;23(4–5):573–611. - PubMed
-
- Kizil C, et al. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2012;72(3):429–61. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

