Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial
- PMID: 26157991
- PMCID: PMC4478785
- DOI: 10.1117/1.NPh.2.2.020801
Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial
Abstract
Functional near-infrared spectroscopy (fNIRS) is an optical imaging method that is used to noninvasively measure cerebral hemoglobin concentration changes induced by brain activation. Using structural guidance in fNIRS research enhances interpretation of results and facilitates making comparisons between studies. AtlasViewer is an open-source software package we have developed that incorporates multiple spatial registration tools to enable structural guidance in the interpretation of fNIRS studies. We introduce the reader to the layout of the AtlasViewer graphical user interface, the folder structure, and user files required in the creation of fNIRS probes containing sources and detectors registered to desired locations on the head, evaluating probe fabrication error and intersubject probe placement variability, and different procedures for estimating measurement sensitivity to different brain regions as well as image reconstruction performance. Further, we detail how AtlasViewer provides a generic head atlas for guiding interpretation of fNIRS results, but also permits users to provide subject-specific head anatomies to interpret their results. We anticipate that AtlasViewer will be a valuable tool in improving the anatomical interpretation of fNIRS studies.
Keywords: atlas; image reconstruction; near-infrared spectroscopy; photon migration; probe design; tutorial.
Figures


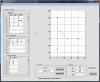
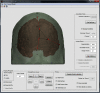
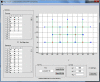
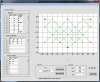


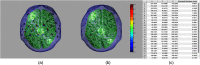

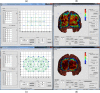

References
-
- Jasper H. H., “The ten-twenty electrode system of the International Federation,” Electroencephalogr. Clin. Neurophysiol. 10, 371–375 (1958). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

