How short is short? Optimum source-detector distance for short-separation channels in functional near-infrared spectroscopy
- PMID: 26158009
- PMCID: PMC4478880
- DOI: 10.1117/1.NPh.2.2.025005
How short is short? Optimum source-detector distance for short-separation channels in functional near-infrared spectroscopy
Abstract
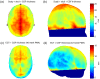
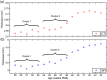
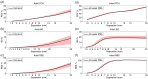
In recent years, it has been demonstrated that using functional near-infrared spectroscopy (fNIRS) channels with short separations to explicitly sample extra-cerebral tissues can provide a significant improvement in the accuracy and reliability of fNIRS measurements. The aim of these short-separation channels is to measure the same superficial hemodynamics observed by standard fNIRS channels while also being insensitive to the brain. We use Monte Carlo simulations of photon transport in anatomically informed multilayer models to determine the optimum source-detector distance for short-separation channels in adult and newborn populations. We present a look-up plot that provides (for an acceptable value of short-separation channel brain sensitivity relative to standard channel brain sensitivity) the optimum short-separation distance. Though values vary across the scalp, when the acceptable ratio of the short-separation channel brain sensitivity to standard channel brain sensitivity is set at 5%, the optimum short-separation distance is 8.4 mm in the typical adult and 2.15 mm in the term-age infant.
Keywords: Monte Carlo simulations; functional near-infrared spectroscopy; short-separation channel; source–detector distance.
Figures



Similar articles
-
Reduction of global interference of scalp-hemodynamics in functional near-infrared spectroscopy using short distance probes.Neuroimage. 2016 Nov 1;141:120-132. doi: 10.1016/j.neuroimage.2016.06.054. Epub 2016 Jun 30. Neuroimage. 2016. PMID: 27374729
-
Short-channel functional near-infrared spectroscopy regressions improve when source-detector separation is reduced.Neurophotonics. 2014 Jul;1(1):015002. doi: 10.1117/1.NPh.1.1.015002. Epub 2014 Jul 15. Neurophotonics. 2014. PMID: 26157972 Free PMC article.
-
The Temporal Confounding Effects of Extra-cerebral Contamination Factors on the Hemodynamic Signal Measured by Functional Near-Infrared Spectroscopy.J Lasers Med Sci. 2019 Fall;10(Suppl 1):S73-S81. doi: 10.15171/jlms.2019.S14. Epub 2019 Dec 1. J Lasers Med Sci. 2019. PMID: 32021678 Free PMC article.
-
Spatial registration for functional near-infrared spectroscopy: from channel position on the scalp to cortical location in individual and group analyses.Neuroimage. 2014 Jan 15;85 Pt 1:92-103. doi: 10.1016/j.neuroimage.2013.07.025. Epub 2013 Jul 25. Neuroimage. 2014. PMID: 23891905 Review.
-
Statistical analysis of fNIRS data: a comprehensive review.Neuroimage. 2014 Jan 15;85 Pt 1:72-91. doi: 10.1016/j.neuroimage.2013.06.016. Epub 2013 Jun 15. Neuroimage. 2014. PMID: 23774396 Review.
Cited by
-
The Potential of Functional Near-Infrared Spectroscopy-Based Neurofeedback-A Systematic Review and Recommendations for Best Practice.Front Neurosci. 2020 Jul 21;14:594. doi: 10.3389/fnins.2020.00594. eCollection 2020. Front Neurosci. 2020. PMID: 32848528 Free PMC article.
-
Awareness of embodiment enhances enjoyment and engages sensorimotor cortices.Hum Brain Mapp. 2024 Jul 15;45(10):e26786. doi: 10.1002/hbm.26786. Hum Brain Mapp. 2024. PMID: 38994692 Free PMC article.
-
In Silico Investigation of SNR and Dermis Sensitivity for Optimum Dual-Channel Near-Infrared Glucose Sensor Designs for Different Skin Colors.Biosensors (Basel). 2022 Sep 29;12(10):805. doi: 10.3390/bios12100805. Biosensors (Basel). 2022. PMID: 36290941 Free PMC article.
-
Brain activation during the N-back working memory task in individuals with spinal cord injury: a functional near-infrared spectroscopy study.bioRxiv [Preprint]. 2024 Feb 12:2024.02.09.579655. doi: 10.1101/2024.02.09.579655. bioRxiv. 2024. PMID: 38405769 Free PMC article. Preprint.
-
Short-channel regression in functional near-infrared spectroscopy is more effective when considering heterogeneous scalp hemodynamics.Neurophotonics. 2020 Jul;7(3):035011. doi: 10.1117/1.NPh.7.3.035011. Epub 2020 Sep 29. Neurophotonics. 2020. PMID: 33029548 Free PMC article.
References
-
- Boas D. A., et al. , “Noninvasive imaging of cerebral activation with iffuse optical tomography,”In Vivo Optical Imaging of Brain Function, Ron D., Ed., 2nd ed., CRC Press, Boca Raton, Florida: (2009). - PubMed

