Single-cell imaging of inflammatory caspase dimerization reveals differential recruitment to inflammasomes
- PMID: 26158519
- PMCID: PMC4650733
- DOI: 10.1038/cddis.2015.186
Single-cell imaging of inflammatory caspase dimerization reveals differential recruitment to inflammasomes
Abstract
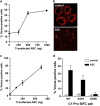
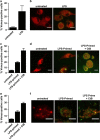
The human inflammatory caspases, including caspase-1, -4, -5 and -12, are considered as key regulators of innate immunity protecting from sepsis and numerous inflammatory diseases. Caspase-1 is activated by proximity-induced dimerization following recruitment to inflammasomes but the roles of the remaining inflammatory caspases in inflammasome assembly are unclear. Here, we use caspase bimolecular fluorescence complementation to visualize the assembly of inflammasomes and dimerization of inflammatory caspases in single cells. We observed caspase-1 dimerization induced by the coexpression of a range of inflammasome proteins and by lipospolysaccharide (LPS) treatment in primary macrophages. Caspase-4 and -5 were only dimerized by select inflammasome proteins, whereas caspase-12 dimerization was not detected by any investigated treatment. Strikingly, we determined that certain inflammasome proteins could induce heterodimerization of caspase-1 with caspase-4 or -5. Caspase-5 homodimerization and caspase-1/-5 heterodimerization was also detected in LPS-primed primary macrophages in response to cholera toxin subunit B. The subcellular localization and organization of the inflammasome complexes varied markedly depending on the upstream trigger and on which caspase or combination of caspases were recruited. Three-dimensional imaging of the ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain)/caspase-1 complexes revealed a large spherical complex of ASC with caspase-1 dimerized on the outer surface. In contrast, NALP1 (NACHT leucine-rich repeat protein 1)/caspase-1 complexes formed large filamentous structures. These results argue that caspase-1, -4 or -5 can be recruited to inflammasomes under specific circumstances, often leading to distinctly organized and localized complexes that may impact the functions of these proteases.
Figures




References
-
- 1Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science 1992; 256: 97–100. - PubMed
-
- 2Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 1997; 386: 619–623. - PubMed
-
- 4Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 2007; 19: 615–622. - PubMed
-
- 5Bertin J, DiStefano PS. The PYRIN domain: a novel motif found in apoptosis and inflammation proteins. Cell Death Differ 2000; 7: 1273–1274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

