Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo
- PMID: 26158531
- PMCID: PMC4497677
- DOI: 10.1371/journal.pone.0132367
Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo
Abstract
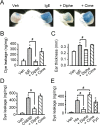
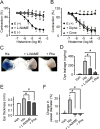
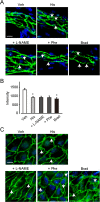
Histamine is a mediator of allergic inflammation released mainly from mast cells. Although histamine strongly increases vascular permeability, its precise mechanism under in vivo situation remains unknown. We here attempted to reveal how histamine induces vascular hyperpermeability focusing on the key regulators of vascular permeability, blood flow and endothelial barrier. Degranulation of mast cells by antigen-stimulation or histamine treatment induced vascular hyperpermeability and tissue swelling in mouse ears. These were abolished by histamine H1 receptor antagonism. Intravital imaging showed that histamine dilated vasculature, increased blood flow, while it induced hyperpermeability in venula. Whole-mount staining showed that histamine disrupted endothelial barrier formation of venula indicated by changes in vascular endothelial cadherin (VE-cadherin) localization at endothelial cell junction. Inhibition of nitric oxide synthesis (NOS) by L-NAME or vasoconstriction by phenylephrine strongly inhibited the histamine-induced blood flow increase and hyperpermeability without changing the VE-cadherin localization. In vitro, measurements of trans-endothelial electrical resistance of human dermal microvascular endothelial cells (HDMECs) showed that histamine disrupted endothelial barrier. Inhibition of protein kinase C (PKC) or Rho-associated protein kinase (ROCK), NOS attenuated the histamine-induced barrier disruption. These observations suggested that histamine increases vascular permeability mainly by nitric oxide (NO)-dependent vascular dilation and subsequent blood flow increase and maybe partially by PKC/ROCK/NO-dependent endothelial barrier disruption.
Conflict of interest statement
Figures



References
-
- Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30(4):573–86. Epub 2008/05/24. . - PubMed
-
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circulation research. 2000;87(4):335–40. Epub 2000/08/19. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

