Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction
- PMID: 26158648
- PMCID: PMC4497655
- DOI: 10.1371/journal.pgen.1005309
Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction
Abstract
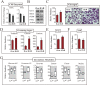
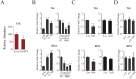
Hematophagous mosquitoes serve as vectors of multiple devastating human diseases, and many unique physiological features contribute to the incredible evolutionary success of these insects. These functions place high-energy demands on a reproducing female mosquito, and carbohydrate metabolism (CM) must be synchronized with these needs. Functional analysis of metabolic gene profiling showed that major CM pathways, including glycolysis, glycogen and sugar metabolism, and citrate cycle, are dramatically repressed at post eclosion (PE) stage in mosquito fat body followed by a sharply increase at post-blood meal (PBM) stage, which were also verified by Real-time RT-PCR. Consistent to the change of transcript and protein level of CM genes, the level of glycogen, glucose and trehalose and other secondary metabolites are also periodically accumulated and degraded during the reproductive cycle respectively. Levels of triacylglycerols (TAG), which represent another important energy storage form in the mosquito fat body, followed a similar tendency. On the other hand, ATP, which is generated by catabolism of these secondary metabolites, showed an opposite trend. Additionally, we used RNA interference studies for the juvenile hormone and ecdysone receptors, Met and EcR, coupled with transcriptomics and metabolomics analyses to show that these hormone receptors function as major regulatory switches coordinating CM with the differing energy requirements of the female mosquito throughout its reproductive cycle. Our study demonstrates how, by metabolic reprogramming, a multicellular organism adapts to drastic and rapid functional changes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

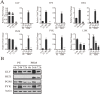
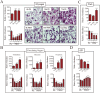

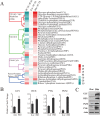
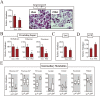
References
-
- Attardo GM, Hansen IA, Raikhel AS (2005) Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol 35: 661–675. - PubMed
-
- Clements AN (1992) The biology of mosquitoes Volume 1: Development, Nutrition and Reproduction. London: Chapman & Hall; 511 p.
-
- Raikhel AS, Kokoza VA, Zhu J, Martin D, Wang SF, et al. (2002) Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochem Mol Biol 32: 1275–1286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

