Structural basis and functional analysis of the SARS coronavirus nsp14-nsp10 complex
- PMID: 26159422
- PMCID: PMC4522806
- DOI: 10.1073/pnas.1508686112
Structural basis and functional analysis of the SARS coronavirus nsp14-nsp10 complex
Abstract
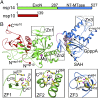
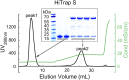
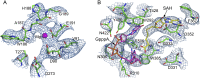
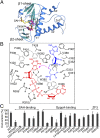
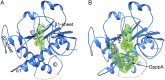
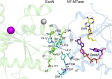
Nonstructural protein 14 (nsp14) of coronaviruses (CoV) is important for viral replication and transcription. The N-terminal exoribonuclease (ExoN) domain plays a proofreading role for prevention of lethal mutagenesis, and the C-terminal domain functions as a (guanine-N7) methyl transferase (N7-MTase) for mRNA capping. The molecular basis of both these functions is unknown. Here, we describe crystal structures of severe acute respiratory syndrome (SARS)-CoV nsp14 in complex with its activator nonstructural protein10 (nsp10) and functional ligands. One molecule of nsp10 interacts with ExoN of nsp14 to stabilize it and stimulate its activity. Although the catalytic core of nsp14 ExoN is reminiscent of proofreading exonucleases, the presence of two zinc fingers sets it apart from homologs. Mutagenesis studies indicate that both these zinc fingers are essential for the function of nsp14. We show that a DEEDh (the five catalytic amino acids) motif drives nucleotide excision. The N7-MTase domain exhibits a noncanonical MTase fold with a rare β-sheet insertion and a peripheral zinc finger. The cap-precursor guanosine-P3-adenosine-5',5'-triphosphate and S-adenosyl methionine bind in proximity in a highly constricted pocket between two β-sheets to accomplish methyl transfer. Our studies provide the first glimpses, to our knowledge, into the architecture of the nsp14-nsp10 complex involved in RNA viral proofreading.
Keywords: CoV; exoribonuclease; methyltransferase; nsp14; proofreading.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- De Groot R, et al. Order nidovirales. In: King AM, Adams MJ, Lefkowitz EJ, editors. Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; London: 2011. pp. 785–795.
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

