Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation
- PMID: 26159653
- PMCID: PMC4766118
- DOI: 10.1208/s12249-015-0360-7
Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation
Abstract
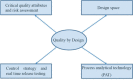
Hot-melt extrusion (HME) is a promising technology for the production of new chemical entities in the developmental pipeline and for improving products already on the market. In drug discovery and development, industry estimates that more than 50% of active pharmaceutical ingredients currently used belong to the biopharmaceutical classification system II (BCS class II), which are characterized as poorly water-soluble compounds and result in formulations with low bioavailability. Therefore, there is a critical need for the pharmaceutical industry to develop formulations that will enhance the solubility and ultimately the bioavailability of these compounds. HME technology also offers an opportunity to earn intellectual property, which is evident from an increasing number of patents and publications that have included it as a novel pharmaceutical formulation technology over the past decades. This review had a threefold objective. First, it sought to provide an overview of HME principles and present detailed engineered extrusion equipment designs. Second, it included a number of published reports on the application of HME techniques that covered the fields of solid dispersions, microencapsulation, taste masking, targeted drug delivery systems, sustained release, films, nanotechnology, floating drug delivery systems, implants, and continuous manufacturing using the wet granulation process. Lastly, this review discussed the importance of using the quality by design approach in drug development, evaluated the process analytical technology used in pharmaceutical HME monitoring and control, discussed techniques used in HME, and emphasized the potential for monitoring and controlling hot-melt technology.
Keywords: hot-melt extrusion; process analytical technology; quality by design; screw design; solid dispersion.
Figures








References
-
- El-Egakey MA, Soliva M, Speiser P. Hot extruded dosage forms. Technology and dissolution kinetics of polymeric matrices. Pharm Acta Helv. 1971;46(1):31–52. - PubMed
-
- Dreiblatt A. Process design. In: Ghebre-Sellassie I, Martin C, editors. Pharmaceutical extrusion technology. New York: Marcel Dekker, Inc; 2003. pp. 153–169.
-
- McGinity JW, Zhang F, Koleng J, Repka M. Hot-melt extrusion as a pharmaceutical process. Am Pharm Rev. 2001;4:25–37.
-
- Shah S, Repka MA. Melt extrusion in drug delivery: three decades of progress. In: Repka MA, Langley N, DiNunzio J, editors. Melt extrusion. New York: Springer; 2013. pp. 3–46.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

