Innate Immune Defenses Mediated by Two ILC Subsets Are Critical for Protection against Acute Clostridium difficile Infection
- PMID: 26159718
- PMCID: PMC4537644
- DOI: 10.1016/j.chom.2015.06.011
Innate Immune Defenses Mediated by Two ILC Subsets Are Critical for Protection against Acute Clostridium difficile Infection
Abstract
Infection with the opportunistic enteric pathogen Clostridium difficile is an increasingly common clinical complication that follows antibiotic treatment-induced gut microbiota perturbation. Innate lymphoid cells (ILCs) are early responders to enteric pathogens; however, their role during C. difficile infection is undefined. To identify immune pathways that mediate recovery from C. difficile infection, we challenged C57BL/6, Rag1(-/-) (which lack T and B cells), and Rag2(-/-)Il2rg(-/-) (Ragγc(-/-)) mice (which additionally lack ILCs) with C. difficile. In contrast to Rag1(-/-) mice, ILC-deficient Ragγc(-/-) mice rapidly succumbed to infection. Rag1(-/-) but not Ragγc(-/-) mice upregulate expression of ILC1- or ILC3-associated proteins following C. difficile infection. Protection against infection was restored by transferring ILCs into Ragγc(-/-) mice. While ILC3s made a minor contribution to resistance, loss of IFN-γ or T-bet-expressing ILC1s in Rag1(-/-) mice increased susceptibility to C. difficile. These data demonstrate a critical role for ILC1s in defense against C. difficile.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

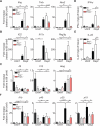
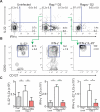


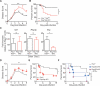
Comment in
-
The Bug Stops Here: Innate Lymphoid Cells in Clostridium difficile Infection.Cell Host Microbe. 2015 Jul 8;18(1):5-6. doi: 10.1016/j.chom.2015.06.015. Cell Host Microbe. 2015. PMID: 26159713
References
-
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. - PubMed
-
- Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nature immunology. 2013;14:221–229. - PubMed
-
- Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. Journal of immunology. 2008;180:7265–7275. - PubMed
-
- Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infection and immunity. 2012;80:62–73. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

