Impact of inactivated poliovirus vaccine on mucosal immunity: implications for the polio eradication endgame
- PMID: 26159938
- PMCID: PMC4673562
- DOI: 10.1586/14760584.2015.1052800
Impact of inactivated poliovirus vaccine on mucosal immunity: implications for the polio eradication endgame
Abstract
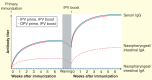
The polio eradication endgame aims to bring transmission of all polioviruses to a halt. To achieve this aim, it is essential to block viral replication in individuals via induction of a robust mucosal immune response. Although it has long been recognized that inactivated poliovirus vaccine (IPV) is incapable of inducing a strong mucosal response on its own, it has recently become clear that IPV may boost immunity in the intestinal mucosa among individuals previously immunized with oral poliovirus vaccine. Indeed, mucosal protection appears to be stronger following a booster dose of IPV than oral poliovirus vaccine, especially in older children. Here, we review the available evidence regarding the impact of IPV on mucosal immunity, and consider the implications of this evidence for the polio eradication endgame. We conclude that the implementation of IPV in both routine and supplementary immunization activities has the potential to play a key role in halting poliovirus transmission, and thereby hasten the eradication of polio.
Keywords: environmental surveillance; eradication; inactivated poliovirus vaccine; mucosal immunity; oral poliovirus vaccine; poliovirus.
Figures
References
-
- Global Polio Eradication Initiative 2013 Polio eradication and endgame strategic plan (2013-2018)
-
- Ghendon YZ, Sanakoyeva II. Comparison of the resistance of the intestinal tract to poliomyelitis virus (Sabin’s strains) in persons after naturally and experimentally acquired immunity. Acta Virol. 1961;5:265–73.
-
- Onorato IM, Modlin JF, McBean AM, et al. Mucosal immunity induced by enhanced-potency inactivated and oral polio vaccines. J Infect Dis. 1991;163:1–6. - PubMed
-
Study comparing the impact of inactivated poliovirus vaccine (IPV) and oral poliovirus vaccine (OPV) on both poliovirus-specific secretory antibodies and shedding following OPV challenge.
-
- Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183(11):6883–92. - PubMed
-
- Hanlon P, Hanlon L, Marsh V, et al. Serological comparisons of approaches to polio vaccination in the Gambia. Lancet. 1987;1:800–1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
