H3K36 methylation promotes longevity by enhancing transcriptional fidelity
- PMID: 26159996
- PMCID: PMC4511212
- DOI: 10.1101/gad.263707.115
H3K36 methylation promotes longevity by enhancing transcriptional fidelity
Abstract
Epigenetic mechanisms, including histone post-translational modifications, control longevity in diverse organisms. Relatedly, loss of proper transcriptional regulation on a global scale is an emerging phenomenon of shortened life span, but the specific mechanisms linking these observations remain to be uncovered. Here, we describe a life span screen in Saccharomyces cerevisiae that is designed to identify amino acid residues of histones that regulate yeast replicative aging. Our results reveal that lack of sustained histone H3K36 methylation is commensurate with increased cryptic transcription in a subset of genes in old cells and with shorter life span. In contrast, deletion of the K36me2/3 demethylase Rph1 increases H3K36me3 within these genes, suppresses cryptic transcript initiation, and extends life span. We show that this aging phenomenon is conserved, as cryptic transcription also increases in old worms. We propose that epigenetic misregulation in aging cells leads to loss of transcriptional precision that is detrimental to life span, and, importantly, this acceleration in aging can be reversed by restoring transcriptional fidelity.
Keywords: H3K36 methylation; aging; cryptic transcription; epigenetics.
© 2015 Sen et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



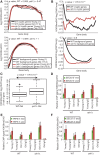

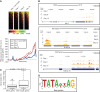
References
Publication types
MeSH terms
Substances
Grants and funding
- R00 AG037646/AG/NIA NIH HHS/United States
- K99/R00AG037646/AG/NIA NIH HHS/United States
- U54GM103250/GM/NIGMS NIH HHS/United States
- P01 AG001751/AG/NIA NIH HHS/United States
- P30AG013280/AG/NIA NIH HHS/United States
- T32ES007032/ES/NIEHS NIH HHS/United States
- P01AG031862/AG/NIA NIH HHS/United States
- R01 AG043080/AG/NIA NIH HHS/United States
- P30 AG013280/AG/NIA NIH HHS/United States
- R01 AG039390/AG/NIA NIH HHS/United States
- P01 AG031862/AG/NIA NIH HHS/United States
- T32 ES007032/ES/NIEHS NIH HHS/United States
- R01AG043080/AG/NIA NIH HHS/United States
- R01AG039390/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
