Characterization of kappa opioid receptor mediated, dynorphin-stimulated [35S]GTPγS binding in mouse striatum for the evaluation of selective KOR ligands in an endogenous setting
- PMID: 26160155
- PMCID: PMC4655192
- DOI: 10.1016/j.neuropharm.2015.07.001
Characterization of kappa opioid receptor mediated, dynorphin-stimulated [35S]GTPγS binding in mouse striatum for the evaluation of selective KOR ligands in an endogenous setting
Abstract
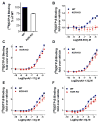
Differential modulation of kappa opioid receptor (KOR) signaling has been a proposed strategy for developing therapies for drug addiction and depression by either activating or blocking this receptor. Hence, there have been significant efforts to generate ligands with diverse pharmacological properties including partial agonists, antagonists, allosteric modulators as well as ligands that selectively activate some pathways while not engaging others (biased agonists). It is becoming increasingly evident that G protein coupled receptor signaling events are context dependent and that what may occur in cell based assays may not be fully indicative of signaling events that occur in the naturally occurring environment. As new ligands are developed, it is important to assess their signaling capacity in relevant endogenous systems in comparison to the performance of endogenous agonists. Since KOR is considered the cognate receptor for dynorphin peptides we have evaluated the selectivity profiles of dynorphin peptides in wild-type (WT), KOR knockout (KOR-KO), and mu opioid receptor knockout (MOR-KO) mice using [35S]GTPγS binding assay in striatal membrane preparations. We find that while the small molecule KOR agonist U69,593, is very selective for KOR, dynorphin peptides promiscuously stimulate G protein signaling in striatum. Furthermore, our studies demonstrate that norBNI and 5'GNTI are highly nonselective antagonists as they maintain full potency and efficacy against dynorphin signaling in the absence of KOR. Characterization of a new KOR antagonist, which may be more selective than NorBNI and 5'GNTI, is presented using this approach.
Keywords: Drug discovery; Dynorphin; KOR antagonist; Kappa opioid receptor; Mouse striatum; Selectivity; [(35)S]GTPγS binding.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
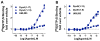





References
-
- Alt A, Mansour A, Akil H, Medzihradsky F, Traynor JR, Woods JH. Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J Pharmacol Exp Ther. 1998;286:282–288. - PubMed
-
- Becker JA, Wallace A, Garzon A, Ingallinella P, Bianchi E, Cortese R, Simonin F, Kieffer BL, Pessi A. Ligands for kappa-opioid and ORL1 receptors identified from a conformationally constrained peptide combinatorial library. J Biol Chem. 1999;274:27513–27522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

