The Steroidogenic Enzyme AKR1C3 Regulates Stability of the Ubiquitin Ligase Siah2 in Prostate Cancer Cells
- PMID: 26160177
- PMCID: PMC4543648
- DOI: 10.1074/jbc.M115.662155
The Steroidogenic Enzyme AKR1C3 Regulates Stability of the Ubiquitin Ligase Siah2 in Prostate Cancer Cells
Abstract
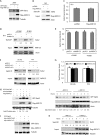
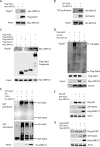
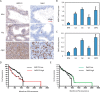
Re-activation of androgen receptor (AR) activity is the main driver for development of castration-resistant prostate cancer. We previously reported that the ubiquitin ligase Siah2 enhanced AR transcriptional activity and prostate cancer cell growth. Among the genes we found to be regulated by Siah2 was AKR1C3, which encodes a key androgen biosynthetic enzyme implicated in castration-resistant prostate cancer development. Here, we found that Siah2 inhibition in CWR22Rv1 prostate cancer cells decreased AKR1C3 expression as well as intracellular androgen levels, concomitant with inhibition of cell growth in vitro and in orthotopic prostate tumors. Re-expression of either wild-type or catalytically inactive forms of AKR1C3 partially rescued AR activity and growth defects in Siah2 knockdown cells, suggesting a nonenzymatic role for AKR1C3 in these outcomes. Unexpectedly, AKR1C3 re-expression in Siah2 knockdown cells elevated Siah2 protein levels, whereas AKR1C3 knockdown had the opposite effect. We further found that AKR1C3 can bind Siah2 and inhibit its self-ubiquitination and degradation, thereby increasing Siah2 protein levels. We observed parallel expression of Siah2 and AKR1C3 in human prostate cancer tissues. Collectively, our findings identify a new role for AKR1C3 in regulating Siah2 stability and thus enhancing Siah2-dependent regulation of AR activity in prostate cancer cells.
Keywords: androgen; androgen receptor; prostate cancer; transcription regulation; ubiquitin ligase.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Siegel R., Naishadham D., Jemal A. (2012) Cancer statistics, 2012. CA-Cancer J. Clin. 62, 10–29 - PubMed
-
- Grasso C. S., Wu Y. M., Robinson D. R., Cao X., Dhanasekaran S. M., Khan A. P., Quist M. J., Jing X., Lonigro R. J., Brenner J. C., Asangani I. A., Ateeq B., Chun S. Y., Siddiqui J., Sam L., et al. (2012) The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 - PMC - PubMed
-
- Antonarakis E. S., Lu C., Wang H., Luber B., Nakazawa M., Roeser J. C., Chen Y., Mohammad T. A., Chen Y., Fedor H. L., Lotan T. L., Zheng Q., De Marzo A. M., Isaacs J. T., Isaacs W. B., et al. (2014) AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

