Initial experience of radiotherapy plus cetuximab for Japanese head and neck cancer patients
- PMID: 26160181
- PMCID: PMC4577007
- DOI: 10.1093/jrr/rrv038
Initial experience of radiotherapy plus cetuximab for Japanese head and neck cancer patients
Abstract
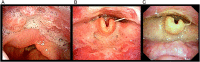
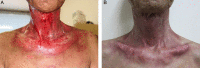
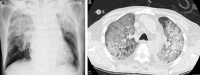
In Japan, cetuximab with concurrent bioradiotherapy (BRT) for squamous cell carcinoma of head and neck (SCCHN) was approved in December 2012. We herein report our initial experience of BRT, with special emphasis on acute toxicities of this combination therapy. Thirty-one non-metastatic SCCHN patients who underwent BRT using cetuximab between July 2013 and June 2014 were retrospectively evaluated. All patients received cetuximab with a loading dose of 400 mg/m(2) one week before the start of radiotherapy, followed by 250 mg/m(2) per week during radiotherapy. The median cycle of cetuximab was seven cycles and the median dose of radiotherapy was 70 Gy. Twenty-five patients (80.6%) accomplished planned radiotherapy and six cycles or more cetuximab administration. Six patients (19.4%) discontinued cetuximab. Grade 3 dermatitis, mucositis and infusion reaction occurred in 19.4%, 48.3% and 3.2%, respectively. One patient experienced Grade 3 gastrointestinal bleeding caused by diverticular hemorrhage during BRT. Grade 3 drug-induced pneumonitis occurred in two patients. The response rate was 74%, including 55% with a complete response. BRT using cetuximab for Japanese patients with SCCHN was feasible as an alternative for cisplatin-based concurrent chemoradiation, although longer follow-up is necessary to evaluate late toxicities.
Keywords: acute toxicity; cetuximab; head and neck cancer; initial experience; radiotherapy.
© The Author 2015. Published by Oxford University Press on behalf of The Japan Radiation Research Society and Japanese Society for Radiation Oncology.
Figures
Similar articles
-
Real-world clinical outcomes in Japanese patients with locally advanced squamous cell carcinoma of the head and neck treated with radiotherapy plus cetuximab: a prospective observational study (JROSG12-2).Int J Clin Oncol. 2022 Nov;27(11):1675-1683. doi: 10.1007/s10147-022-02228-3. Epub 2022 Aug 24. Int J Clin Oncol. 2022. PMID: 36001247
-
Comparison of acute toxicities associated with cetuximab-based bioradiotherapy and platinum-based chemoradiotherapy for head and neck squamous cell carcinomas: A single-institution retrospective study in Japan.Acta Otolaryngol. 2015 Aug;135(8):853-8. doi: 10.3109/00016489.2015.1030772. Epub 2015 Mar 26. Acta Otolaryngol. 2015. PMID: 25814008
-
The efficiency and adverse events of radiotherapy with cetuximab for Japanese head and neck cancer patients.Auris Nasus Larynx. 2017 Dec;44(6):724-728. doi: 10.1016/j.anl.2017.01.005. Epub 2017 Feb 22. Auris Nasus Larynx. 2017. PMID: 28237712
-
Toxicities Following Stereotactic Ablative Radiotherapy Treatment of Locally-Recurrent and Previously Irradiated Head and Neck Squamous Cell Carcinoma.Semin Radiat Oncol. 2016 Apr;26(2):112-9. doi: 10.1016/j.semradonc.2015.11.007. Epub 2015 Nov 27. Semin Radiat Oncol. 2016. PMID: 27000507 Review.
-
Treatment of head and neck cancers: issues for clinical pharmacists.Pharmacotherapy. 2009 May;29(5):578-92. doi: 10.1592/phco.29.5.578. Pharmacotherapy. 2009. PMID: 19397465 Review.
Cited by
-
Real-world clinical outcomes in Japanese patients with locally advanced squamous cell carcinoma of the head and neck treated with radiotherapy plus cetuximab: a prospective observational study (JROSG12-2).Int J Clin Oncol. 2022 Nov;27(11):1675-1683. doi: 10.1007/s10147-022-02228-3. Epub 2022 Aug 24. Int J Clin Oncol. 2022. PMID: 36001247
-
Safety and efficacy of concurrent carboplatin or cetuximab plus radiotherapy for locally advanced head and neck cancer patients ineligible for treatment with cisplatin.Int J Clin Oncol. 2019 May;24(5):468-475. doi: 10.1007/s10147-018-01392-9. Epub 2019 Jan 17. Int J Clin Oncol. 2019. PMID: 30656463 Clinical Trial.
-
Retrospective evaluation of concomitant cetuximab and radiotherapy tolerance for locoregional advanced head and neck squamous cell carcinoma treatment in patients unfit for platinum-based chemotherapy.Eur Arch Otorhinolaryngol. 2017 Jul;274(7):2883-2889. doi: 10.1007/s00405-017-4550-7. Epub 2017 Apr 5. Eur Arch Otorhinolaryngol. 2017. PMID: 28382396
References
-
- Katanoda K, Matsuda T, Matsuda A, et al. An updated report of the trends in cancer incidence and mortality in Japan. Jpn J Clin Oncol 2013;43:492–507. - PubMed
-
- Matsuda T, Marugame T, Kamo K-I, et al. Cancer incidence and incidence rates in Japan in 2006: based on data from 15 population-based cancer registries in the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2012;42:139–47. - PubMed
-
- Pignon J-P, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. - PubMed
-
- Pignon J, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet 2000;355:949–55. - PubMed
-
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160–74. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

