Oligonucleotide Therapies: The Past and the Present
- PMID: 26160334
- PMCID: PMC4554547
- DOI: 10.1089/hum.2015.070
Oligonucleotide Therapies: The Past and the Present
Abstract
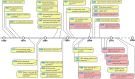
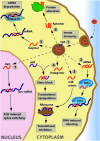
In this review we address the development of oligonucleotide (ON) medicines from a historical perspective by listing the landmark discoveries in this field. The various biological processes that have been targeted and the corresponding ON interventions found in the literature are discussed together with brief updates on some of the more recent developments. Most ON therapies act through antisense mechanisms and are directed against various RNA species, as exemplified by gapmers, steric block ONs, antagomirs, small interfering RNAs (siRNAs), micro-RNA mimics, and splice switching ONs. However, ONs binding to Toll-like receptors and those forming aptamers have completely different modes of action. Similar to other novel medicines, the path to success has been lined with numerous failures, where different therapeutic ONs did not stand the test of time. Since the first ON drug was approved for clinical use in 1998, the therapeutic landscape has changed considerably, but many challenges remain until the expectations for this new form of medicine are met. However, there is room for cautious optimism.
Figures
References
-
- Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 1953;171:737–738 - PubMed
-
- Reist EJ, Benitez A, Goodman L. The synthesis of some 5'-thiopentofuranosylpyrimidines. J Org Chem 1964;29:554–558
-
- Codington JF, Doerr IL, Fox JJ. Nucleosides. XVIII. Synthesis of 2′-fluorothymidine, 2′-fluorodeoxyuridine, and other 2′-halogeno-2′-deoxy nucleosides. J Org Chem 1964;29:558–564
-
- Eckstein F. Nucleoside phosphorothioates. J Am Chem Soc 1966;88:4292–4294 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
