Pattern recognition receptors in microbial keratitis
- PMID: 26160532
- PMCID: PMC4815674
- DOI: 10.1038/eye.2015.118
Pattern recognition receptors in microbial keratitis
Abstract
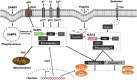
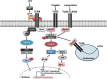
Microbial keratitis is a significant cause of global visual impairment and blindness. Corneal infection can be caused by a wide variety of pathogens, each of which exhibits a range of mechanisms by which the immune system is activated. The complexity of the immune response to corneal infection is only now beginning to be elucidated. Crucial to the cornea's defences are the pattern-recognition receptors: Toll-like and Nod-like receptors and the subsequent activation of inflammatory pathways. These inflammatory pathways include the inflammasome and can lead to significant tissue destruction and corneal damage, with the potential for resultant blindness. Understanding the immune mechanisms behind this tissue destruction may enable improved identification of therapeutic targets to aid development of more specific therapies for reducing corneal damage in infectious keratitis. This review summarises current knowledge of pattern-recognition receptors and their downstream pathways in response to the major keratitis-causing organisms and alludes to potential therapeutic approaches that could alleviate corneal blindness.
Figures
Similar articles
-
Signaling pathways downstream of pattern-recognition receptors and their cross talk.Annu Rev Biochem. 2007;76:447-80. doi: 10.1146/annurev.biochem.76.060605.122847. Annu Rev Biochem. 2007. PMID: 17328678 Review.
-
Toll-like receptors in ocular surface disease.Exp Eye Res. 2010 Jun;90(6):679-87. doi: 10.1016/j.exer.2010.03.012. Epub 2010 Mar 24. Exp Eye Res. 2010. PMID: 20346359 Free PMC article. Review.
-
NOD-Like Receptors in Infection, Immunity, and Diseases.Yonsei Med J. 2016 Jan;57(1):5-14. doi: 10.3349/ymj.2016.57.1.5. Yonsei Med J. 2016. PMID: 26632377 Free PMC article. Review.
-
Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors.J Allergy Clin Immunol. 2012 Jan;129(1):14-24; quiz 25-6. doi: 10.1016/j.jaci.2011.11.004. J Allergy Clin Immunol. 2012. PMID: 22196521 Free PMC article. Review.
-
Toll-like receptors and NOD-like receptors: domain architecture and cellular signalling.Adv Exp Med Biol. 2009;653:48-57. doi: 10.1007/978-1-4419-0901-5_4. Adv Exp Med Biol. 2009. PMID: 19799111 Review.
Cited by
-
Living with your biome: how the bacterial microbiome impacts ocular surface health and disease.Expert Rev Ophthalmol. 2024;19(2):89-103. doi: 10.1080/17469899.2024.2306582. Epub 2024 Feb 4. Expert Rev Ophthalmol. 2024. PMID: 38764699 Free PMC article.
-
The Growing Threat of Gonococcal Blindness.Antibiotics (Basel). 2018 Jul 12;7(3):59. doi: 10.3390/antibiotics7030059. Antibiotics (Basel). 2018. PMID: 30002340 Free PMC article.
-
Ocular Surface Infection Mediated Molecular Stress Responses: A Review.Int J Mol Sci. 2022 Mar 14;23(6):3111. doi: 10.3390/ijms23063111. Int J Mol Sci. 2022. PMID: 35328532 Free PMC article. Review.
-
MyD88 contribution to ocular surface homeostasis.PLoS One. 2017 Aug 10;12(8):e0182153. doi: 10.1371/journal.pone.0182153. eCollection 2017. PLoS One. 2017. PMID: 28796783 Free PMC article.
-
Bacterial keratitis: Photodynamic inactivation reduced experimental inflammation.Exp Ther Med. 2017 Nov;14(5):4509-4514. doi: 10.3892/etm.2017.5109. Epub 2017 Sep 5. Exp Ther Med. 2017. PMID: 29104658 Free PMC article.
References
-
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012; 96: 614–618. - PubMed
-
- Wang H, Zhang Y, Li Z, Wang T, Liu P. Prevalence and causes of corneal blindness. Clin Experiment Ophthalmol 2014; 42: 249–253. - PubMed
-
- Dart JKG. The epidemiology of contact lens related disease in the United Kingdom: a review. CLAO J 1993; 19: 241–246. - PubMed
-
- Erie JC, Nevitt MP, Hodge DO, Ballard DJ. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol 1993; 111: 1665–1671. - PubMed
-
- Jeng BH, Gritz DC, Kumar AB, Holsclaw DS, Porco TC, Smith SD et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol 2010; 128: 1022–1028. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

