Iron homeostasis in host defence and inflammation
- PMID: 26160612
- PMCID: PMC4801113
- DOI: 10.1038/nri3863
Iron homeostasis in host defence and inflammation
Abstract
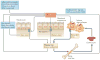
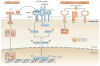
Iron is an essential trace element for multicellular organisms and nearly all microorganisms. Although iron is abundant in the environment, common forms of iron are minimally soluble and therefore poorly accessible to biological organisms. Microorganisms entering a mammalian host face multiple mechanisms that further restrict their ability to obtain iron and thereby limit their pathogenicity. Iron levels also modulate host defence, as iron content in macrophages regulates their cytokine production. Here, we review recent advances that highlight the role of systemic and cellular iron-regulating mechanisms in protecting hosts from infection, emphasizing aspects that are applicable to human health and disease.
Conflict of interest statement
The authors declare competing interests: see Web version for details.
Figures

References
-
- Weinberg ED. Nutritional immunity: host’s attempt to withhold iron from microbial invaders. JAMA. 1975;231:39–41. - PubMed
-
- Kim S, Ponka P. Nitric oxide-mediated modulation of iron regulatory proteins: implication for cellular iron homeostasis. Blood Cells Mol Dis. 2002;29:400–410. - PubMed
-
- Viatte L, Grone HJ, Hentze MW, Galy B. In vivo role(s) of the iron regulatory proteins (IRP) 1 and 2 in aseptic local inflammation. J Mol Med. 2009;87:913–921. - PubMed
-
- Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338:768–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

