Presynaptic active zones in invertebrates and vertebrates
- PMID: 26160654
- PMCID: PMC4552486
- DOI: 10.15252/embr.201540434
Presynaptic active zones in invertebrates and vertebrates
Abstract
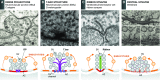
The regulated release of neurotransmitter occurs via the fusion of synaptic vesicles (SVs) at specialized regions of the presynaptic membrane called active zones (AZs). These regions are defined by a cytoskeletal matrix assembled at AZs (CAZ), which functions to direct SVs toward docking and fusion sites and supports their maturation into the readily releasable pool. In addition, CAZ proteins localize voltage-gated Ca(2+) channels at SV release sites, bringing the fusion machinery in close proximity to the calcium source. Proteins of the CAZ therefore ensure that vesicle fusion is temporally and spatially organized, allowing for the precise and reliable release of neurotransmitter. Importantly, AZs are highly dynamic structures, supporting presynaptic remodeling, changes in neurotransmitter release efficacy, and thus presynaptic forms of plasticity. In this review, we discuss recent advances in the study of active zones, highlighting how the CAZ molecularly defines sites of neurotransmitter release, endocytic zones, and the integrity of synapses.
Keywords: active zone; cytoskeletal matrix; fusion; release; synaptic vesicle.
© 2015 The Authors.
Figures




References
-
- Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 2004;19:262–270. - PubMed
-
- Jin Y, Garner CC. Molecular mechanisms of presynaptic differentiation. Annu Rev Cell Dev Biol. 2008;24:237–262. - PubMed
-
- Zhen M, Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

