A Novel, Stable, Estradiol-Stimulating, Osteogenic Yam Protein with Potential for the Treatment of Menopausal Syndrome
- PMID: 26160710
- PMCID: PMC5155516
- DOI: 10.1038/srep10179
A Novel, Stable, Estradiol-Stimulating, Osteogenic Yam Protein with Potential for the Treatment of Menopausal Syndrome
Erratum in
-
Erratum: A Novel, Stable, Estradiol-Stimulating, Osteogenic Yam Protein with Potential for the Treatment of Menopausal Syndrome.Sci Rep. 2015 Dec 18;5:17129. doi: 10.1038/srep17129. Sci Rep. 2015. PMID: 26681303 Free PMC article. No abstract available.
Abstract
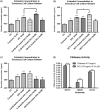
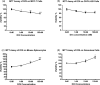
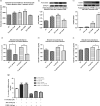
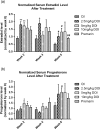
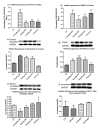
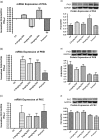
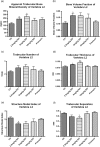
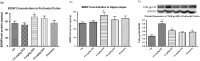
A novel protein, designated as DOI, isolated from the Chinese yam (Dioscorea opposita Thunb.) could be the first protein drug for the treatment of menopausal syndrome and an alternative to hormone replacement therapy (HRT), which is known to have undesirable side effects. DOI is an acid- and thermo-stable protein with a distinctive N-terminal sequence Gly-Ile-Gly-Lys-Ile-Thr-Thr-Tyr-Trp-Gly-Gln-Tyr-Ser-Asp-Glu-Pro-Ser-Leu-Thr-Glu. DOI was found to stimulate estradiol biosynthesis in rat ovarian granulosa cells; induce estradiol and progesterone secretion in 16- to 18-month-old female Sprague Dawley rats by upregulating expressions of follicle-stimulating hormone receptor and ovarian aromatase; counteract the progression of osteoporosis and augment bone mineral density; and improve cognitive functioning by upregulating protein expressions of brain-derived neurotrophic factor and TrkB receptors in the prefrontal cortex. Furthermore, DOI did not stimulate the proliferation of breast cancer and ovarian cancer cells, which suggest it could be a more efficacious and safer alternative to HRT.
Conflict of interest statement
Findings from this study have been used to apply for patents (Patent Cooperation Treaty Patent Pub. No.: WO/2013/023617; U.S. Patent Pub. No.: US20130217626 A1; European Patent Pub. No.: EP 2750684 A1; China Patent pub. No.: CN103945854 A)
Figures


Similar articles
-
Comparative Analysis of Proteins with Stimulating Activity on Ovarian Estradiol Biosynthesis from Four Different Dioscorea Species in vitro Using Both Phenotypic and Target-based Approaches: Implication for Treating Menopause.Appl Biochem Biotechnol. 2016 Sep;180(1):79-93. doi: 10.1007/s12010-016-2084-x. Epub 2016 Apr 30. Appl Biochem Biotechnol. 2016. PMID: 27131879
-
Mutual regulation of growth hormone and bone morphogenetic protein system in steroidogenesis by rat granulosa cells.Endocrinology. 2012 Jan;153(1):469-80. doi: 10.1210/en.2011-1646. Epub 2011 Nov 8. Endocrinology. 2012. PMID: 22067323
-
Role of ERK1/2 in the differential synthesis of progesterone and estradiol by granulosa cells.Biochem Biophys Res Commun. 2001 Dec 14;289(4):796-800. doi: 10.1006/bbrc.2001.6052. Biochem Biophys Res Commun. 2001. PMID: 11735115
-
A novel antagonistic effect of the bone morphogenetic protein system on prolactin actions in regulating steroidogenesis by granulosa cells.Endocrinology. 2010 Nov;151(11):5506-18. doi: 10.1210/en.2010-0265. Epub 2010 Sep 1. Endocrinology. 2010. PMID: 20810564
-
Research and Development of Proteins and Peptides with Therapeutic Potential from Yam Tubers.Curr Protein Pept Sci. 2019;20(3):277-284. doi: 10.2174/1389203719666180622094356. Curr Protein Pept Sci. 2019. PMID: 29932033 Review.
Cited by
-
Identification of Steroidogenic Components Derived From Gardenia jasminoides Ellis Potentially Useful for Treating Postmenopausal Syndrome.Front Pharmacol. 2018 May 30;9:390. doi: 10.3389/fphar.2018.00390. eCollection 2018. Front Pharmacol. 2018. PMID: 29899696 Free PMC article.
-
Ameliorating effect of Erxian decoction combined with Fructus Schisandrae chinensis (Wu Wei Zi) on menopausal sweating and serum hormone profiles in a rat model.Chin Med. 2016 Nov 22;11:47. doi: 10.1186/s13020-016-0117-6. eCollection 2016. Chin Med. 2016. PMID: 27895702 Free PMC article.
-
A new osteogenic protein isolated from Dioscorea opposita Thunb accelerates bone defect healing through the mTOR signaling axis.Bioact Mater. 2023 Apr 23;27:429-446. doi: 10.1016/j.bioactmat.2023.04.018. eCollection 2023 Sep. Bioact Mater. 2023. PMID: 37152710 Free PMC article.
-
An analysis of the combination frequencies of constituent medicinal herbs in prescriptions for the treatment of bone and joint disorder in Korean medicine: determination of a group of candidate prescriptions for universal use.Integr Med Res. 2017 Dec;6(4):344-353. doi: 10.1016/j.imr.2017.09.001. Epub 2017 Sep 19. Integr Med Res. 2017. PMID: 29296561 Free PMC article.
-
A Review of the Science of Colorful, Plant-Based Food and Practical Strategies for "Eating the Rainbow".J Nutr Metab. 2019 Jun 2;2019:2125070. doi: 10.1155/2019/2125070. eCollection 2019. J Nutr Metab. 2019. PMID: 33414957 Free PMC article. Review.
References
-
- Avis N. E. et al. Is there a menopausal syndrom? Menopausal status and symptoms across racial/ethnic groups. Soc. Sci. Med. 52, 345–356, doi:S0277953600001477 [pii] (2001). - PubMed
-
- Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organ. Tech. Rep. Ser. 866, 1–107 (1996). - PubMed
-
- Barnabei V. M. et al. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet. Gynecol. 100, 1209–1218, doi:S0029784402023694 [pii] (2002). - PubMed
-
- Buist D. S. et al. Hormone therapy prescribing patterns in the United States. Obstet. Gynecol. 104, 1042–1050, doi:104/5/1042 [pii]10.1097/01.AOG.0000143826.38439.af (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

