Bamboo leaf extract improves spatial learning ability in a rat model with senile dementia
- PMID: 26160717
- PMCID: PMC4506950
- DOI: 10.1631/jzus.B1400249
Bamboo leaf extract improves spatial learning ability in a rat model with senile dementia
Abstract
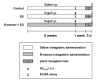
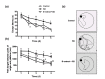
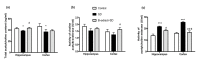
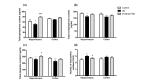
Senile dementia (SD) is a syndrome characterized by progressive neurological deterioration. Treatment for the disease is still under investigation. Bamboo leaf extract (B-extract) has been known for its biological efficacy in anti-oxidant and anti-cancer activities. However, study on B-extract for its protection against dementia is very limited. The effect of B-extract on a rat model with SD was examined. B-extract improved spatial learning ability of the dementia rats. The hippocampus of dementia model rats showed reduced levels of acetylcholine (ACh), epinephrine (E), norepinephrine (NE), and dopamine (DA), and increased activities of acetylcholine esterase (AChE) and monoamine oxidase (MAO). Treatment with B-extract 20 mg/(kg·d) for 7 weeks significantly inhibited the enzyme activity compared with untreated dementia rats, and raised the levels of ACh, E, and DA in the hippocampus. In addition, treatment with B-extract elevated the level of γ-aminobutyric acid (GABA), but reduced the level of glutamate (Glu) in the brain. These data suggest that B-extract might be a potential drug in treating impairment of spatial memory in dementia rats by regulating the central neurotransmitter function.
Keywords: Alzheimer’s disease; Bamboo leaf extract; Dementia; Neurotransmitter.
Conflict of interest statement
All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures
Similar articles
-
Role of Eclipta prostrata extract in improving spatial learning and memory deficits in D-galactose-induced aging in rats.J Tradit Chin Med. 2019 Oct;39(5):649-657. J Tradit Chin Med. 2019. PMID: 32186114
-
Effect of some medicinal plant extracts on the oxidative stress status in Alzheimer's disease induced in rats.Eur Rev Med Pharmacol Sci. 2012 Jul;16 Suppl 3:31-42. Eur Rev Med Pharmacol Sci. 2012. PMID: 22957416
-
Sasa quelpaertensis leaf extract improves high fat diet-induced lipid abnormalities and regulation of lipid metabolism genes in rats.J Med Food. 2014 May;17(5):571-81. doi: 10.1089/jmf.2013.2916. Epub 2014 Apr 16. J Med Food. 2014. PMID: 24738745
-
Noradrenergic-cholinergic interaction: its possible role in memory dysfunction associated with senile dementia.Arch Gerontol Geriatr Suppl. 1989;1:99-108. Arch Gerontol Geriatr Suppl. 1989. PMID: 2569317 Review.
-
Final report on the safety assessment of AloeAndongensis Extract, Aloe Andongensis Leaf Juice,aloe Arborescens Leaf Extract, Aloe Arborescens Leaf Juice, Aloe Arborescens Leaf Protoplasts, Aloe Barbadensis Flower Extract, Aloe Barbadensis Leaf, Aloe Barbadensis Leaf Extract, Aloe Barbadensis Leaf Juice,aloe Barbadensis Leaf Polysaccharides, Aloe Barbadensis Leaf Water, Aloe Ferox Leaf Extract, Aloe Ferox Leaf Juice, and Aloe Ferox Leaf Juice Extract.Int J Toxicol. 2007;26 Suppl 2:1-50. doi: 10.1080/10915810701351186. Int J Toxicol. 2007. PMID: 17613130 Review.
Cited by
-
Insights and progress on the biosynthesis, metabolism, and physiological functions of gamma-aminobutyric acid (GABA): a review.PeerJ. 2024 Dec 16;12:e18712. doi: 10.7717/peerj.18712. eCollection 2024. PeerJ. 2024. PMID: 39703920 Free PMC article. Review.
-
Interhemispheric functional connectivity for Alzheimer's disease and amnestic mild cognitive impairment based on the triple network model.J Zhejiang Univ Sci B. 2018 Dec.;19(12):924-934. doi: 10.1631/jzus.B1800381. J Zhejiang Univ Sci B. 2018. PMID: 30507076 Free PMC article.
-
Effects of bamboo leaf extract on energy metabolism, antioxidant capacity, and biogenesis of small intestine mitochondria in broilers.J Anim Sci. 2023 Jan 3;101:skac391. doi: 10.1093/jas/skac391. J Anim Sci. 2023. PMID: 36440554 Free PMC article.
-
Protein phosphatase 2A activators reverse age-related behavioral changes by targeting neural cell senescence.Aging Cell. 2023 Mar;22(3):e13780. doi: 10.1111/acel.13780. Epub 2023 Jan 16. Aging Cell. 2023. PMID: 36644807 Free PMC article.
-
The alleviative effects comparison of four flavonoids from bamboo leaves on ulcerative colitis in an Alzheimer mouse model.CNS Neurosci Ther. 2024 Feb;30(2):e14620. doi: 10.1111/cns.14620. CNS Neurosci Ther. 2024. PMID: 38334213 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

