Lovastatin overcomes gefitinib resistance through TNF-α signaling in human cholangiocarcinomas with different LKB1 statuses in vitro and in vivo
- PMID: 26160843
- PMCID: PMC4695157
- DOI: 10.18632/oncotarget.4408
Lovastatin overcomes gefitinib resistance through TNF-α signaling in human cholangiocarcinomas with different LKB1 statuses in vitro and in vivo
Abstract
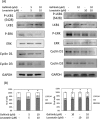
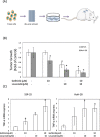
Gefitinib resistance has been shown to complicate cancer therapy. Lovastatin is a proteasome inhibitor that enhances gefitinib-induced antiproliferation in non-small cell lung cancer. The objective of this study is to investigate the mechanism of lovastatin-induced antiproliferation in gefitinib-resistant human cholangiocarcinoma.Two gefitinib-resistant cholangiocarcinoma cell lines, SSP-25 and HuH-28, were used in this study to determine how to compensate gefitinib resistance. The combined effect of these two drugs was examined using the MTT assay, qPCR, immunoblotting, flow cytometry, and in vivo xenograft. Results indicated that lovastatin enhanced TNF-α-induced cell death in vitro. In addition, the combination of lovastatin with gefitinib enhanced accumulation of TNF-α. Furthermore, the treatment induced a synergistic cytotoxic effect and antiproliferation through apoptosis in SSP-25 cells and cell cycle arrest in HuH-28 cells. Reproductive results were also observed in in vivo xenografts. These observations suggest that the combination of gefitinib and lovastatin might have additive antiproliferative effects against gefitinib-resistant cholangiocarcinoma cells. Based on these observations, we concluded that the combination of gefitinib and lovastatin could be used to overcome gefitinib resistance in cholangiocarcinoma cells.
Keywords: cholangiocarcinomas; combination therapy; gefitinib; lovastatin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Combination effect between bortezomib and tumor necrosis factor alpha on gefitinib-resistant non-small cell lung cancer cell lines.Anticancer Res. 2009 Jun;29(6):2315-22. Anticancer Res. 2009. PMID: 19528497
-
Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines.Clin Cancer Res. 2013 Jul 1;19(13):3508-19. doi: 10.1158/1078-0432.CCR-12-2777. Epub 2013 May 21. Clin Cancer Res. 2013. PMID: 23695170
-
Lovastatin overcomes gefitinib resistance in human non-small cell lung cancer cells with K-Ras mutations.Invest New Drugs. 2010 Dec;28(6):791-9. doi: 10.1007/s10637-009-9319-4. Epub 2009 Sep 17. Invest New Drugs. 2010. PMID: 19760159
-
Targeting cancer stem cells in cholangiocarcinoma (Review).Int J Oncol. 2020 Aug;57(2):397-408. doi: 10.3892/ijo.2020.5074. Epub 2020 May 28. Int J Oncol. 2020. PMID: 32468022 Free PMC article. Review.
-
Novel investigational therapies for treating biliary tract carcinoma.Expert Opin Investig Drugs. 2016 Dec;25(12):1423-1436. doi: 10.1080/13543784.2016.1252330. Epub 2016 Nov 3. Expert Opin Investig Drugs. 2016. PMID: 27771967 Review.
Cited by
-
The Chemosensory Function of Primary Cilia Regulates Cholangiocyte Migration, Invasion, and Tumor Growth.Hepatology. 2019 Apr;69(4):1582-1598. doi: 10.1002/hep.30308. Epub 2019 Mar 15. Hepatology. 2019. PMID: 30299561 Free PMC article.
-
The Mevalonate Pathway, a Metabolic Target in Cancer Therapy.Front Oncol. 2021 Feb 25;11:626971. doi: 10.3389/fonc.2021.626971. eCollection 2021. Front Oncol. 2021. PMID: 33718197 Free PMC article. Review.
-
Fluvastatin potentiates anticancer activity of vorinostat in renal cancer cells.Cancer Sci. 2020 Jan;111(1):112-126. doi: 10.1111/cas.14225. Epub 2019 Nov 25. Cancer Sci. 2020. PMID: 31675763 Free PMC article.
-
Review and Application of Integrin Alpha v Beta 6 in the Diagnosis and Treatment of Cholangiocarcinoma and Pancreatic Ductal Adenocarcinoma.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231189399. doi: 10.1177/15330338231189399. Technol Cancer Res Treat. 2023. PMID: 37525872 Free PMC article. Review.
-
NDAT Targets PI3K-Mediated PD-L1 Upregulation to Reduce Proliferation in Gefitinib-Resistant Colorectal Cancer.Cells. 2020 Aug 3;9(8):1830. doi: 10.3390/cells9081830. Cells. 2020. PMID: 32756527 Free PMC article.
References
-
- Leone F, Cavalloni G, Pignochino Y, Sarotto I, Ferraris R, Piacibello W, Venesio T, Capussotti L, Risio M, Aglietta M. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:1680–1685. - PubMed
-
- Hsu M, Sasaki M, Igarashi S, Sato Y, Nakanuma Y. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer. 2013;119:1669–1674. - PubMed
-
- Wu CC, Yu CT, Chang GC, Lai JM, Hsu SL. Aurora-A promotes gefitinib resistance via a NF-kappaB signaling pathway in p53 knockdown lung cancer cells. Biochemical and biophysical research communications. 2011;405:168–172. - PubMed
-
- Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, Liu DD, Bekele BN, Herbst RS, Wistuba II. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:2890–2896. - PubMed
-
- Daines WP, Rajagopalan V, Grossbard ML, Kozuch P. Gallbladder and biliary tract carcinoma: A comprehensive update, Part 2. Oncology. 2004;18:1049–1059. discussion 1060, 1065-1046, 1068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

