ErbB-3 activation by NRG-1β sustains growth and promotes vemurafenib resistance in BRAF-V600E colon cancer stem cells (CSCs)
- PMID: 26160848
- PMCID: PMC4627280
- DOI: 10.18632/oncotarget.4642
ErbB-3 activation by NRG-1β sustains growth and promotes vemurafenib resistance in BRAF-V600E colon cancer stem cells (CSCs)
Abstract
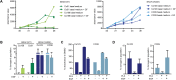
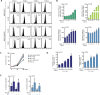
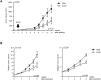
Approximately 5-10% of metastatic colorectal cancers harbor a BRAF-V600E mutation, which is correlated with resistance to EGFR-targeted therapies and worse clinical outcome. Vice versa, targeted inhibition of BRAF-V600E with the selective inhibitor PLX 4032 (Vemurafenib) is severely limited due to feedback re-activation of EGFR in these tumors. Mounting evidence indicates that upregulation of the ErbB-3 signaling axis may occur in response to several targeted therapeutics, including Vemurafenib, and NRG-1β-dependent re-activation of the PI3K/AKT survival pathway has been associated with therapy resistance.Here we show that colon CSCs express, next to EGFR and ErbB-2, also significant amounts of ErbB-3 on their membrane. This expression is functional as NRG-1β strongly induces AKT/PKB and ERK phosphorylation, cell proliferation, clonogenic growth and promotes resistance to Vemurafenib in BRAF-V600E mutant colon CSCs. This resistance was completely dependent on ErbB-3 expression, as evidenced by knockdown of ErbB-3. More importantly, resistance could be alleviated with therapeutic antibody blocking ErbB-3 activation, which impaired NRG-1β-driven AKT/PKB and ERK activation, clonogenic growth in vitro and tumor growth in xenograft models. In conclusion, our findings suggest that targeting ErbB-3 receptors could represent an effective therapeutic approach in BRAF-V600E mutant colon cancer.
Keywords: ErbB-3; NRG-1β; colon cancer stem cells; vemurafenib.
Conflict of interest statement
GS is an employee and shareholders of Mediapharma s.r.l.. SI is president and founder of Mediapharma s.r.l.. The other authors have no potential conflict of interest to disclose.
Figures

Similar articles
-
Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR.Nature. 2012 Jan 26;483(7387):100-3. doi: 10.1038/nature10868. Nature. 2012. PMID: 22281684
-
Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents.Clin Cancer Res. 2013 Feb 1;19(3):657-67. doi: 10.1158/1078-0432.CCR-11-1446. Epub 2012 Dec 18. Clin Cancer Res. 2013. PMID: 23251002 Free PMC article.
-
Metastasis-associated MCL1 and P16 copy number alterations dictate resistance to vemurafenib in a BRAFV600E patient-derived papillary thyroid carcinoma preclinical model.Oncotarget. 2015 Dec 15;6(40):42445-67. doi: 10.18632/oncotarget.6442. Oncotarget. 2015. PMID: 26636651 Free PMC article.
-
Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma.Clin Ther. 2012 Jul;34(7):1474-86. doi: 10.1016/j.clinthera.2012.06.009. Epub 2012 Jun 27. Clin Ther. 2012. PMID: 22742884 Review.
-
Vemurafenib.Recent Results Cancer Res. 2014;201:215-25. doi: 10.1007/978-3-642-54490-3_13. Recent Results Cancer Res. 2014. PMID: 24756795 Review.
Cited by
-
ErbB3 Phosphorylation as Central Event in Adaptive Resistance to Targeted Therapy in Metastatic Melanoma: Early Detection in CTCs during Therapy and Insights into Regulation by Autocrine Neuregulin.Cancers (Basel). 2019 Sep 25;11(10):1425. doi: 10.3390/cancers11101425. Cancers (Basel). 2019. PMID: 31557826 Free PMC article.
-
ADAM10-mediated release of heregulin confers resistance to trastuzumab by activating HER3.Oncotarget. 2016 Mar 1;7(9):10243-54. doi: 10.18632/oncotarget.7200. Oncotarget. 2016. PMID: 26863569 Free PMC article.
-
Prevalence of HER3 Expression in Pancreatic Cancer Patients Treated With Systemic Chemotherapy.Cancer Med. 2024 Dec;13(23):e70474. doi: 10.1002/cam4.70474. Cancer Med. 2024. PMID: 39651731 Free PMC article.
-
Targeting HER3 for cancer treatment: a new horizon for an old target.ESMO Open. 2023 Feb;8(1):100790. doi: 10.1016/j.esmoop.2023.100790. Epub 2023 Feb 8. ESMO Open. 2023. PMID: 36764093 Free PMC article. Review.
-
Resistance to antibody-drug conjugates: A review.Acta Pharm Sin B. 2025 Feb;15(2):737-756. doi: 10.1016/j.apsb.2024.12.036. Epub 2024 Dec 31. Acta Pharm Sin B. 2025. PMID: 40177568 Free PMC article. Review.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Roskoski R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. - PubMed
-
- Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

