Ty1 retrovirus-like element Gag contains overlapping restriction factor and nucleic acid chaperone functions
- PMID: 26160887
- PMCID: PMC4551931
- DOI: 10.1093/nar/gkv695
Ty1 retrovirus-like element Gag contains overlapping restriction factor and nucleic acid chaperone functions
Abstract
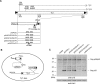
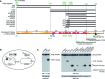
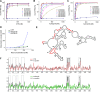
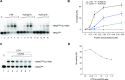
Ty1 Gag comprises the capsid of virus-like particles and provides nucleic acid chaperone (NAC) functions during retrotransposition in budding yeast. A subgenomic Ty1 mRNA encodes a truncated Gag protein (p22) that is cleaved by Ty1 protease to form p18. p22/p18 strongly inhibits transposition and can be considered an element-encoded restriction factor. Here, we show that only p22 and its short derivatives restrict Ty1 mobility whereas other regions of GAG inhibit mobility weakly if at all. Mutational analyses suggest that p22/p18 is synthesized from either of two closely spaced AUG codons. Interestingly, AUG1p18 and AUG2p18 proteins display different properties, even though both contain a region crucial for RNA binding and NAC activity. AUG1p18 shows highly reduced NAC activity but specific binding to Ty1 RNA, whereas AUG2p18 shows the converse behavior. p22/p18 affects RNA encapsidation and a mutant derivative defective for RNA binding inhibits the RNA chaperone activity of the C-terminal region (CTR) of Gag-p45. Moreover, affinity pulldowns show that p18 and the CTR interact. These results support the idea that one aspect of Ty1 restriction involves inhibition of Gag-p45 NAC functions by p22/p18-Gag interactions.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Similar articles
-
A trans-dominant form of Gag restricts Ty1 retrotransposition and mediates copy number control.J Virol. 2015 Apr;89(7):3922-38. doi: 10.1128/JVI.03060-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609815 Free PMC article.
-
Structure of Ty1 Internally Initiated RNA Influences Restriction Factor Expression.Viruses. 2017 Apr 10;9(4):74. doi: 10.3390/v9040074. Viruses. 2017. PMID: 28394277 Free PMC article.
-
The Ty1 Retrotransposon Restriction Factor p22 Targets Gag.PLoS Genet. 2015 Oct 9;11(10):e1005571. doi: 10.1371/journal.pgen.1005571. eCollection 2015 Oct. PLoS Genet. 2015. PMID: 26451601 Free PMC article.
-
A self-encoded capsid derivative restricts Ty1 retrotransposition in Saccharomyces.Curr Genet. 2016 May;62(2):321-9. doi: 10.1007/s00294-015-0550-6. Epub 2015 Dec 9. Curr Genet. 2016. PMID: 26650614 Free PMC article. Review.
-
Nucleic acid chaperone activity of retroviral Gag proteins.RNA Biol. 2010 Nov-Dec;7(6):700-5. doi: 10.4161/rna.7.6.13685. Epub 2010 Nov 1. RNA Biol. 2010. PMID: 21045546 Free PMC article. Review.
Cited by
-
Co-option of a non-retroviral endogenous viral element in planthoppers.Nat Commun. 2023 Nov 9;14(1):7264. doi: 10.1038/s41467-023-43186-2. Nat Commun. 2023. PMID: 37945658 Free PMC article.
-
Ty1 escapes restriction by the self-encoded factor p22 through mutations in capsid.Mob Genet Elements. 2016 Mar 7;6(2):e1154639. doi: 10.1080/2159256X.2016.1154639. eCollection 2016 Mar-Apr. Mob Genet Elements. 2016. PMID: 27141327 Free PMC article.
-
An interchangeable prion-like domain is required for Ty1 retrotransposition.bioRxiv [Preprint]. 2023 Feb 27:2023.02.27.530227. doi: 10.1101/2023.02.27.530227. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 Jul 25;120(30):e2303358120. doi: 10.1073/pnas.2303358120. PMID: 36909481 Free PMC article. Updated. Preprint.
-
Co-option of endogenous viral sequences for host cell function.Curr Opin Virol. 2017 Aug;25:81-89. doi: 10.1016/j.coviro.2017.07.021. Epub 2017 Aug 16. Curr Opin Virol. 2017. PMID: 28818736 Free PMC article. Review.
-
The Mediator co-activator complex regulates Ty1 retromobility by controlling the balance between Ty1i and Ty1 promoters.PLoS Genet. 2018 Feb 20;14(2):e1007232. doi: 10.1371/journal.pgen.1007232. eCollection 2018 Feb. PLoS Genet. 2018. PMID: 29462141 Free PMC article.
References
-
- Voytas D.F., Boeke J.D. Ty1 and Ty5 of Saccharomyces cerevisiae. In: Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. Mobile DNA II. Washington, DC: ASM Press; 2002. pp. 614–630.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

