Regulator of G-protein signaling 2 (RGS2) suppresses premature calcium release in mouse eggs
- PMID: 26160904
- PMCID: PMC4529029
- DOI: 10.1242/dev.121707
Regulator of G-protein signaling 2 (RGS2) suppresses premature calcium release in mouse eggs
Abstract
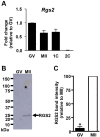
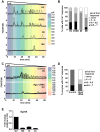
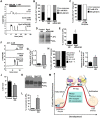
During oocyte maturation, capacity and sensitivity of Ca(2+) signaling machinery increases dramatically, preparing the metaphase II (MII)-arrested egg for fertilization. Upon sperm-egg fusion, Ca(2+) release from IP3-sensitive endoplasmic reticulum stores results in cytoplasmic Ca(2+) oscillations that drive egg activation and initiate early embryo development. Premature Ca(2+) release can cause parthenogenetic activation prior to fertilization; thus, preventing inappropriate Ca(2+) signaling is crucial for ensuring robust MII arrest. Here, we show that regulator of G-protein signaling 2 (RGS2) suppresses Ca(2+) release in MII eggs. Rgs2 mRNA was recruited for translation during oocyte maturation, resulting in ∼ 20-fold more RGS2 protein in MII eggs than in fully grown immature oocytes. Rgs2-siRNA-injected oocytes matured to MII; however, they had increased sensitivity to low pH and acetylcholine (ACh), which caused inappropriate Ca(2+) release and premature egg activation. When matured in vitro, RGS2-depleted eggs underwent spontaneous Ca(2+) increases that were sufficient to cause premature zona pellucida conversion. Rgs2(-/-) females had reduced litter sizes, and their eggs had increased sensitivity to low pH and ACh. Rgs2(-/-) eggs also underwent premature zona pellucida conversion in vivo. These findings indicate that RGS2 functions as a brake to suppress premature Ca(2+) release in eggs that are poised on the brink of development.
Keywords: Calcium; Egg activation; Gq; Meiotic maturation; Oocyte; RGS2.
© 2015. Published by The Company of Biologists Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

