Cln1 gene disruption in mice reveals a common pathogenic link between two of the most lethal childhood neurodegenerative lysosomal storage disorders
- PMID: 26160911
- PMCID: PMC4572073
- DOI: 10.1093/hmg/ddv266
Cln1 gene disruption in mice reveals a common pathogenic link between two of the most lethal childhood neurodegenerative lysosomal storage disorders
Abstract
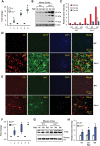
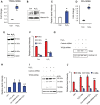
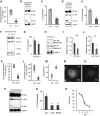
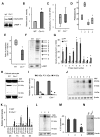
Neurodegeneration is a devastating manifestation in the majority of >50 lysosomal storage disorders (LSDs). Neuronal ceroid lipofuscinoses (NCLs) are the most common childhood neurodegenerative LSDs. Mutations in 13 different genes (called CLNs) underlie various types of NCLs, of which the infantile NCL (INCL) and congenital NCL (CNCL) are the most lethal. Although inactivating mutations in the CLN1 gene encoding palmitoyl-protein thioesterase-1 (PPT1) cause INCL, those in the CLN10 gene encoding cathepsin D (CD) underlie CNCL. PPT1 is a lysosomal thioesterase that cleaves the thioester linkage in S-acylated proteins required for their degradation by lysosomal hydrolases like CD. Thus, PPT1 deficiency causes lysosomal accumulation of these lipidated proteins (major constituents of ceroid) leading to INCL. We sought to determine whether there is a common pathogenic link between INCL and CNCL. Using biochemical, histological and confocal microscopic analyses of brain tissues and cells from Cln1(-/-) mice that mimic INCL, we uncovered that Cln10/CD is overexpressed. Although synthesized in the endoplasmic reticulum, the CD-precursor protein (pro-CD) is transported through endosome to the lysosome where it is proteolytically processed to enzymatically active-CD. We found that despite Cln10 overexpression, the maturation of pro-CD to enzymatically active-CD in lysosome was disrupted. This defect impaired lysosomal degradative function causing accumulation of undegraded cargo in lysosome leading to INCL. Notably, treatment of intact Cln1(-/-) mice as well as cultured brain cells derived from these animals with a thioesterase-mimetic small molecule, N-tert-butyl-hydroxylamine, ameliorated the CD-processing defect. Our findings are significant in that they define a pathway in which Cln1 mutations disrupt the maturation of a major degradative enzyme in lysosome contributing to neuropathology in INCL and suggest that lysosomal CD deficiency is a common pathogenic link between INCL and CNCL.
Published by Oxford University Press 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures


Similar articles
-
Cln3-mutations underlying juvenile neuronal ceroid lipofuscinosis cause significantly reduced levels of Palmitoyl-protein thioesterases-1 (Ppt1)-protein and Ppt1-enzyme activity in the lysosome.J Inherit Metab Dis. 2019 Sep;42(5):944-954. doi: 10.1002/jimd.12106. Epub 2019 May 14. J Inherit Metab Dis. 2019. PMID: 31025705 Free PMC article.
-
Cln1-mutations suppress Rab7-RILP interaction and impair autophagy contributing to neuropathology in a mouse model of infantile neuronal ceroid lipofuscinosis.J Inherit Metab Dis. 2020 Sep;43(5):1082-1101. doi: 10.1002/jimd.12242. Epub 2020 Apr 27. J Inherit Metab Dis. 2020. PMID: 32279353 Free PMC article.
-
Ppt1-deficiency dysregulates lysosomal Ca++ homeostasis contributing to pathogenesis in a mouse model of CLN1 disease.J Inherit Metab Dis. 2022 May;45(3):635-656. doi: 10.1002/jimd.12485. Epub 2022 Mar 17. J Inherit Metab Dis. 2022. PMID: 35150145 Free PMC article.
-
Developmental changes in the expression of neuronal ceroid lipofuscinoses-linked proteins.Mol Genet Metab. 2000 Sep-Oct;71(1-2):190-4. doi: 10.1006/mgme.2000.3071. Mol Genet Metab. 2000. PMID: 11001810 Review.
-
Pathogenesis and therapies for infantile neuronal ceroid lipofuscinosis (infantile CLN1 disease).Biochim Biophys Acta. 2013 Nov;1832(11):1906-9. doi: 10.1016/j.bbadis.2013.05.026. Epub 2013 Jun 6. Biochim Biophys Acta. 2013. PMID: 23747979 Free PMC article. Review.
Cited by
-
Regulation of Death Receptor Signaling by S-Palmitoylation and Detergent-Resistant Membrane Micro Domains-Greasing the Gears of Extrinsic Cell Death Induction, Survival, and Inflammation.Cancers (Basel). 2021 May 21;13(11):2513. doi: 10.3390/cancers13112513. Cancers (Basel). 2021. PMID: 34063813 Free PMC article. Review.
-
Mice deficient in the lysosomal enzyme palmitoyl-protein thioesterase 1 (PPT1) display a complex retinal phenotype.Sci Rep. 2019 Oct 2;9(1):14185. doi: 10.1038/s41598-019-50726-8. Sci Rep. 2019. PMID: 31578378 Free PMC article.
-
An innovative hematopoietic stem cell gene therapy approach benefits CLN1 disease in the mouse model.EMBO Mol Med. 2023 Apr 11;15(4):e15968. doi: 10.15252/emmm.202215968. Epub 2023 Mar 6. EMBO Mol Med. 2023. PMID: 36876653 Free PMC article.
-
Enzymatic activity of palmitoyl-protein thioesterase-1 in serum from schizophrenia significantly associates with schizophrenia diagnosis scales.J Cell Mol Med. 2019 Sep;23(9):6512-6518. doi: 10.1111/jcmm.14496. Epub 2019 Jul 3. J Cell Mol Med. 2019. PMID: 31270934 Free PMC article.
-
Identification of substrates of palmitoyl protein thioesterase 1 highlights roles of depalmitoylation in disulfide bond formation and synaptic function.PLoS Biol. 2022 Mar 31;20(3):e3001590. doi: 10.1371/journal.pbio.3001590. eCollection 2022 Mar. PLoS Biol. 2022. PMID: 35358180 Free PMC article.
References
-
- de Duve C. (1983) Lysosomes revisited. Eur. J. Biochem., 137, 391–397. - PubMed
-
- Harris H., Rubinzstein D.C. (2011) Control of autophagy as a therapy for neurodegenerative disease. Nat. Rev. Neurol., 8, 108–117. - PubMed
-
- Nixon R.A. (2013) The role of autophagy in neurodegenerative disease. Nat. Med., 19, 983–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

