ORGANIC SYNTHESIS. Response to Comment on "Asymmetric syntheses of sceptrin and massadine and evidence for biosynthetic enantiodivergence"
- PMID: 26160939
- PMCID: PMC4536548
- DOI: 10.1126/science.aaa9626
ORGANIC SYNTHESIS. Response to Comment on "Asymmetric syntheses of sceptrin and massadine and evidence for biosynthetic enantiodivergence"
Abstract
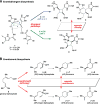
Sherman et al. commented on the precedence of enantiodivergence, listing a number of congeneric natural products with opposite chirality. However, these "congeners" are not derived from enantiodivergent biosyntheses. Instead, they are antipodes arising from separate enantiomeric biosyntheses. A distinct feature of the biosynthesis of the cyclic pyrrole-imidazole dimers is the production of antipodal congeners without the corresponding enantiomers.
Copyright © 2015, American Association for the Advancement of Science.
Figures

Comment on
-
Asymmetric syntheses of sceptrin and massadine and evidence for biosynthetic enantiodivergence.Science. 2014 Oct 10;346(6206):219-24. doi: 10.1126/science.1255677. Science. 2014. PMID: 25301624 Free PMC article.
-
ORGANIC SYNTHESIS. Comment on "Asymmetric syntheses of sceptrin and massadine and evidence for biosynthetic enantiodivergence".Science. 2015 Jul 10;349(6244):149. doi: 10.1126/science.aaa9349. Epub 2015 Jul 9. Science. 2015. PMID: 26160938 Free PMC article.
References
-
- Xia ZQ, Costa MA, Pélissier HC, Davin LB, Lewis NG. J Biol Chem. 2001;276:12614–12623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

