Comparison of Different Cytokine Conditions Reveals Resveratrol as a New Molecule for Ex Vivo Cultivation of Cord Blood-Derived Hematopoietic Stem Cells
- PMID: 26160960
- PMCID: PMC4542867
- DOI: 10.5966/sctm.2014-0284
Comparison of Different Cytokine Conditions Reveals Resveratrol as a New Molecule for Ex Vivo Cultivation of Cord Blood-Derived Hematopoietic Stem Cells
Abstract
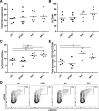
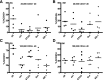
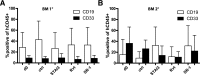
Human cord blood (CB)-derived hematopoietic stem cells (HSCs) are an interesting source for HSC transplantation. However, the number of collected CB-HSCs is often too low for one transplantation; therefore, ex vivo expansion of CB-HSCs is desirable. Current expansion protocols are based on the use of cytokine combinations, including insulin-like growth factor-binding protein 2 (IGFBP2) and angiopoietin-like proteins, or combinations with "small molecules" such as stemregenin-1. The aim of our project was to compare the potential of different CB-HSC expansion strategies side-by-side by phenotypical analysis in vitro and serial engraftment properties in NOD/SCID/IL2rg-/- (NSG) immunodeficient mice. We further identified resveratrol, a naturally occurring polyphenol, as a new, alternative small molecule combined with cytokines to facilitate serum-free ex vivo expansion of human CB-HSCs. The cultivation in resveratrol preserved the CB-HSC phenotype in vitro most efficiently and was ∼2 times more potent than commonly used cytokine conditions (including stem cell factor, thrombopoietin, Fms-related tyrosine kinase 3 ligand, interleukin-6) and the recently established serum-free culture, including IGFBP2 and angiopoietin-like 5. Serial transplantation studies further confirmed resveratrol to support robust multilineage engraftment in primary and secondary NSG recipients. Therefore, our work proposes resveratrol as a new small molecule for improved ex vivo culture and modification of human HSCs based on an efficient ex vivo propagation of the HSC fate.
Significance: Human cord blood (CB)-derived hematopoietic stem cells (HSCs) are an important source for HSC transplantations but restricted in their usage because of their low numbers. In gene therapy, modifications of HSCs relies on their ex vivo modification without losing their stemness properties. Therefore, ex vivo cultivation and expansion of CB-HSCs is important for their effective application in HSC transplantation and gene therapy. Several promising protocols for serum-free cultivation of HSCs using different combinations of cytokines or so-called small molecules are described. A direct comparison was performed of three described serum-free cytokine conditions, demonstrating that the natural occurring polyphenol resveratrol is able to support ex vivo cultivation of CB-HSCs. The results show that resveratrol is an additional candidate for improving ex vivo cultures of HSCs for transplantation and gene therapeutic applications in the future.
Keywords: Cord blood; Expansion; Hematopoietic stem cell; Resveratrol; Serial transplantation.
©AlphaMed Press.
Figures


Similar articles
-
Cotransplantation of ex vivo expanded and unexpanded cord blood units in immunodeficient mice using insulin growth factor binding protein-2-augmented mesenchymal cell cocultures.Biol Blood Marrow Transplant. 2012 May;18(5):674-82. doi: 10.1016/j.bbmt.2012.01.001. Epub 2012 Jan 9. Biol Blood Marrow Transplant. 2012. PMID: 22240732
-
Hematopoietic repopulating ability of CD34⁺ progenitor cells ex vivo expanded with different cytokine combinations.Artif Cells Nanomed Biotechnol. 2015;43(6):398-402. doi: 10.3109/21691401.2014.897630. Epub 2014 Mar 25. Artif Cells Nanomed Biotechnol. 2015. PMID: 24665846
-
Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation.Blood. 2008 Apr 1;111(7):3415-23. doi: 10.1182/blood-2007-11-122119. Epub 2008 Jan 17. Blood. 2008. PMID: 18202223 Free PMC article.
-
Advances in umbilical cord blood stem cell expansion and clinical translation.Exp Hematol. 2015 Jul;43(7):498-513. doi: 10.1016/j.exphem.2015.04.011. Epub 2015 May 10. Exp Hematol. 2015. PMID: 25970610 Review.
-
Ex vivo expansion and engraftment potential of cord blood-derived CD34+ cells in NOD/SCID mice.Ann N Y Acad Sci. 2001 Jun;938:9-17. doi: 10.1111/j.1749-6632.2001.tb03569.x. Ann N Y Acad Sci. 2001. PMID: 11458530 Review.
Cited by
-
Effect of Small Molecule on ex vivo Expansion of Cord Blood Hematopoietic Stem Cells: A Concise Review.Front Cell Dev Biol. 2021 Apr 9;9:649115. doi: 10.3389/fcell.2021.649115. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33898442 Free PMC article. Review.
-
The quest for the holy grail: overcoming challenges in expanding human hematopoietic stem cells for clinical use.Stem Cell Investig. 2023 Jul 11;10:15. doi: 10.21037/sci-2023-016. eCollection 2023. Stem Cell Investig. 2023. PMID: 37457748 Free PMC article. Review.
-
Enhanced self-renewal of human long-term hematopoietic stem cells by a sulfamoyl benzoate derivative targeting p18INK4C.Blood Adv. 2021 Sep 14;5(17):3362-3372. doi: 10.1182/bloodadvances.2020004054. Blood Adv. 2021. PMID: 34477819 Free PMC article.
-
Functional Integrity and Gene Expression Profiles of Human Cord Blood-Derived Hematopoietic Stem and Progenitor Cells Generated In Vitro.Stem Cells Transl Med. 2018 Aug;7(8):602-614. doi: 10.1002/sctm.18-0013. Epub 2018 Apr 26. Stem Cells Transl Med. 2018. PMID: 29701016 Free PMC article.
-
Effects of adipose derived stem cells pretreated with resveratrol on sciatic nerve regeneration in rats.Sci Rep. 2023 Apr 10;13(1):5812. doi: 10.1038/s41598-023-32906-9. Sci Rep. 2023. PMID: 37037844 Free PMC article.
References
-
- Rocha V, Wagner JE, Jr, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846–1854. - PubMed
-
- Rocha V, Gluckman E. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(suppl 1):34–41. - PubMed
-
- Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. - PubMed
-
- Garderet L, Dulphy N, Douay C, et al. Polyclonal and naive but completely formed repertoire the umbilical cord blood alphabeta T-cell repertoire: Characteristics of a polyclonal and naive but completely formed repertoire. Blood. 1998;91:340–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

