IL-33 Aggravates DSS-Induced Acute Colitis in Mouse Colon Lamina Propria by Enhancing Th2 Cell Responses
- PMID: 26161006
- PMCID: PMC4464679
- DOI: 10.1155/2015/913041
IL-33 Aggravates DSS-Induced Acute Colitis in Mouse Colon Lamina Propria by Enhancing Th2 Cell Responses
Abstract
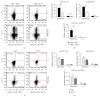
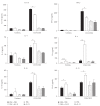
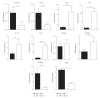
Interleukin- (IL-) 33, a member of the IL-1 cytokine family, is an important modulator of the immune system associated with several immune-mediated diseases. IL-33 was expressed in high level on epithelial cells of intestinal tract. It suggested that IL-33 plays a potential role in inflammatory bowel diseases (IBD). We investigated the role of interleukin- (IL-) 33 in dextran sulphate sodium- (DSS-) induced acute colitis in mice using recombinant mouse IL-33 protein (rIL-33). We found that DSS-induced acute colitis was aggravated by rIL-33 treatment. rIL-33-treated DSS mice showed markedly reduced levels of interferon- (IFN-)γ and IL-17A in their colon lamina propria lymphocytes (LPL), but the levels of Th2 cytokines, such as IL-5 and IL-13, in these cells were significantly increased, compared to DSS mice treated with PBS. Our results suggested that IL-33 stimulated CD4(+)T cells and caused the cell to adopt a Th2-type response but at the same time suppressed Th17 and Th1 cell responses. Therefore, IL-33 may be involved in pathogenesis of DSS-induced acute colitis by promoting Th2 cell response in intestinal mucosa of mice. Modulation of IL-33/ST2 signaling by monoclonal antibody (mAb) could be a novel biological therapy in DSS-induced acute colitis.
Figures


Similar articles
-
IL-33 alleviates DSS-induced chronic colitis in C57BL/6 mice colon lamina propria by suppressing Th17 cell response as well as Th1 cell response.Int Immunopharmacol. 2015 Dec;29(2):846-853. doi: 10.1016/j.intimp.2015.08.032. Epub 2015 Sep 8. Int Immunopharmacol. 2015. PMID: 26359542
-
IL-33 induces both regulatory B cells and regulatory T cells in dextran sulfate sodium-induced colitis.Int Immunopharmacol. 2017 May;46:38-47. doi: 10.1016/j.intimp.2017.02.006. Epub 2017 Feb 28. Int Immunopharmacol. 2017. PMID: 28258042
-
Interleukin-36β exacerbates DSS-induce acute colitis via inhibiting Foxp3+ regulatory T cell response and increasing Th2 cell response.Int Immunopharmacol. 2022 Jul;108:108762. doi: 10.1016/j.intimp.2022.108762. Epub 2022 Apr 15. Int Immunopharmacol. 2022. PMID: 35436743
-
The role of Interleukin-38 in modulating T cells in chronic Colitis: A mouse model study.Cytokine. 2024 Dec;184:156769. doi: 10.1016/j.cyto.2024.156769. Epub 2024 Sep 28. Cytokine. 2024. PMID: 39342821
-
IL-21/IL-21R signaling suppresses intestinal inflammation induced by DSS through regulation of Th responses in lamina propria in mice.Sci Rep. 2016 Aug 22;6:31881. doi: 10.1038/srep31881. Sci Rep. 2016. PMID: 27545302 Free PMC article.
Cited by
-
Loss of interleukin 33 expression in colonic crypts - a potential marker for disease remission in ulcerative colitis.Sci Rep. 2016 Oct 17;6:35403. doi: 10.1038/srep35403. Sci Rep. 2016. PMID: 27748438 Free PMC article.
-
IL-33 Drives Expansion of Type 2 Innate Lymphoid Cells and Regulatory T Cells and Protects Mice From Severe, Acute Colitis.Front Immunol. 2021 Jul 15;12:669787. doi: 10.3389/fimmu.2021.669787. eCollection 2021. Front Immunol. 2021. PMID: 34335571 Free PMC article.
-
IL-33 Prevents MLD-STZ Induction of Diabetes and Attenuate Insulitis in Prediabetic NOD Mice.Front Immunol. 2018 Nov 15;9:2646. doi: 10.3389/fimmu.2018.02646. eCollection 2018. Front Immunol. 2018. PMID: 30498495 Free PMC article.
-
Macrophage orchestration of epithelial and stromal cell homeostasis in the intestine.J Leukoc Biol. 2022 Aug;112(2):313-331. doi: 10.1002/JLB.3RU0322-176R. Epub 2022 May 20. J Leukoc Biol. 2022. PMID: 35593111 Free PMC article. Review.
-
IL-33 Effect on Quantitative Changes of CD4+CD25highFOXP3+ Regulatory T Cells in Children with Type 1 Diabetes.Mediators Inflamm. 2016;2016:9429760. doi: 10.1155/2016/9429760. Epub 2016 Sep 28. Mediators Inflamm. 2016. PMID: 27761063 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

