Sulforaphane Reverses the Expression of Various Tumor Suppressor Genes by Targeting DNMT3B and HDAC1 in Human Cervical Cancer Cells
- PMID: 26161119
- PMCID: PMC4487331
- DOI: 10.1155/2015/412149
Sulforaphane Reverses the Expression of Various Tumor Suppressor Genes by Targeting DNMT3B and HDAC1 in Human Cervical Cancer Cells
Abstract
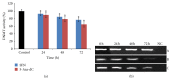
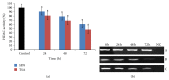
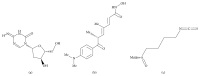
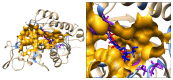
Sulforaphane (SFN) may hinder carcinogenesis by altering epigenetic events in the cells; however, its molecular mechanisms are unclear. The present study investigates the role of SFN in modifying epigenetic events in human cervical cancer cells, HeLa. HeLa cells were treated with SFN (2.5 µM) for a period of 0, 24, 48, and 72 hours for all experiments. After treatment, expressions of DNMT3B, HDAC1, RARβ, CDH1, DAPK1, and GSTP1 were studied using RT-PCR while promoter DNA methylation of tumor suppressor genes (TSGs) was studied using MS-PCR. Inhibition assays of DNA methyl transferases (DNMTs) and histone deacetylases (HDACs) were performed at varying time points. Molecular modeling and docking studies were performed to explore the possible interaction of SFN with HDAC1 and DNMT3B. Time-dependent exposure to SFN decreases the expression of DNMT3B and HDAC1 and significantly reduces the enzymatic activity of DNMTs and HDACs. Molecular modeling data suggests that SFN may interact directly with DNMT3B and HDAC1 which may explain the inhibitory action of SFN. Interestingly, time-dependent reactivation of the studied TSGs via reversal of methylation in SFN treated cells correlates well with its impact on the epigenetic alterations accumulated during cancer development. Thus, SFN may have significant implications for epigenetic based therapy.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

