Unified Modeling of Familial Mediterranean Fever and Cryopyrin Associated Periodic Syndromes
- PMID: 26161132
- PMCID: PMC4464681
- DOI: 10.1155/2015/893507
Unified Modeling of Familial Mediterranean Fever and Cryopyrin Associated Periodic Syndromes
Abstract
Familial mediterranean fever (FMF) and Cryopyrin associated periodic syndromes (CAPS) are two prototypical hereditary autoinflammatory diseases, characterized by recurrent episodes of fever and inflammation as a result of mutations in MEFV and NLRP3 genes encoding Pyrin and Cryopyrin proteins, respectively. Pyrin and Cryopyrin play key roles in the multiprotein inflammasome complex assembly, which regulates activity of an enzyme, Caspase 1, and its target cytokine, IL-1β. Overproduction of IL-1β by Caspase 1 is the main cause of episodic fever and inflammatory findings in FMF and CAPS. We present a unifying dynamical model for FMF and CAPS in the form of coupled nonlinear ordinary differential equations. The model is composed of two subsystems, which capture the interactions and dynamics of the key molecular players and the insults on the immune system. One of the subsystems, which contains a coupled positive-negative feedback motif, captures the dynamics of inflammation formation and regulation. We perform a comprehensive bifurcation analysis of the model and show that it exhibits three modes, capturing the Healthy, FMF, and CAPS cases. The mutations in Pyrin and Cryopyrin are reflected in the values of three parameters in the model. We present extensive simulation results for the model that match clinical observations.
Figures
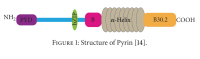










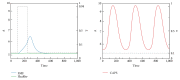




Similar articles
-
Immunology in clinic review series; focus on autoinflammatory diseases: role of inflammasomes in autoinflammatory syndromes.Clin Exp Immunol. 2012 Mar;167(3):382-90. doi: 10.1111/j.1365-2249.2011.04535.x. Clin Exp Immunol. 2012. PMID: 22288581 Free PMC article. Review.
-
Dysregulated production of interleukin-1β upon activation of the NLRP3 inflammasome in patients with familial Mediterranean fever.Hum Immunol. 2015 Jul;76(7):488-95. doi: 10.1016/j.humimm.2015.06.007. Epub 2015 Jun 12. Hum Immunol. 2015. PMID: 26074413
-
Increased NLRP3-dependent interleukin 1β secretion in patients with familial Mediterranean fever: correlation with MEFV genotype.Ann Rheum Dis. 2014 Feb;73(2):462-9. doi: 10.1136/annrheumdis-2012-202774. Epub 2013 Mar 16. Ann Rheum Dis. 2014. PMID: 23505242
-
Familial Mediterranean fever mutations are hypermorphic mutations that specifically decrease the activation threshold of the Pyrin inflammasome.Rheumatology (Oxford). 2018 Jan 1;57(1):100-111. doi: 10.1093/rheumatology/kex373. Rheumatology (Oxford). 2018. PMID: 29040788
-
[Pathophysiological mechanisms underlying cryopyrin-associated periodic syndromes: genetic and molecular basis and the inflammasome].Med Clin (Barc). 2011 Jan;136 Suppl 1:22-8. doi: 10.1016/S0025-7753(11)70005-7. Med Clin (Barc). 2011. PMID: 21596183 Review. Spanish.
Cited by
-
Dynamics of Inflammatory Response in Autoinflammatory Disorders: Autonomous and Hyperinflammatory States.Front Immunol. 2018 Oct 17;9:2422. doi: 10.3389/fimmu.2018.02422. eCollection 2018. Front Immunol. 2018. PMID: 30386349 Free PMC article. Review.
-
Are follistatin-like protein 1 and follistatin-like protein 3 associated with inflammatory processes in patients with familial Mediterranean fever?North Clin Istanb. 2023 Jun 6;10(3):306-313. doi: 10.14744/nci.2022.54189. eCollection 2023. North Clin Istanb. 2023. PMID: 37435280 Free PMC article.
-
The Genetic Heterogeneity among Different Mouse Strains Impacts the Lung Injury Potential of Multiwalled Carbon Nanotubes.Small. 2017 Sep;13(33):10.1002/smll.201700776. doi: 10.1002/smll.201700776. Epub 2017 Jul 5. Small. 2017. PMID: 28677920 Free PMC article.
-
A narrative review on the role of cytokines in the pathogenesis and treatment of familial Mediterranean fever: an emphasis on pediatric cases.Front Pediatr. 2024 Jul 26;12:1421353. doi: 10.3389/fped.2024.1421353. eCollection 2024. Front Pediatr. 2024. PMID: 39132307 Free PMC article. Review.
-
The importance of Mediterranean fever gene in familial Mediterranean fever.Eur J Rheumatol. 2020 Jul 21;7(4):173-6. doi: 10.5152/eurjrheum.2020.20107. Online ahead of print. Eur J Rheumatol. 2020. PMID: 32716837 Free PMC article.
References
-
- Galeazzi M., Gasbarrini G., Ghirardello A., et al. Autoinflammatory syndromes. Clinical and Experimental Rheumatology. 2006;24(40):S79–S85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

